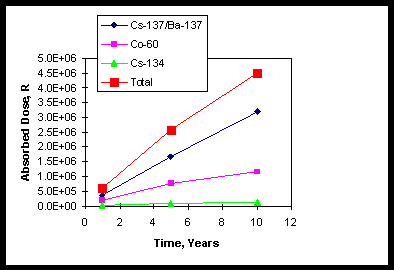
Figure 1. Absorbed Dose Versus Time for Atucha I Resin.
WSRC-TR-97-00338
Radiation Studies with Argentine Ion Exchange Material
Charles L. Crawford
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/help/index.asp
Available electronically at http://www.osti.gov/bridge
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Keywords: Irradiation, Organics, Low Level Wastes, Glass, Vitrification, Resin
Summary
A recent technology exchange between Argentine Nuclear Energy Commission (CNEA) and the US Department of Energy involved vitrification studies of ion exchange resins. Several investigations pertaining to radiation effects on aqueous resin slurries were conducted. Calculations of expected accumulated radiation doses for up to ten years storage were performed. Calculations were based on radionuclide contents of actual Argentine resin samples that were analyzed by the Argentines to contain Cs-137, Co-60 and Cs-134. The accumulated absorbed dose from one Argentine spent resin slurry was calculated to be about 4.5 Mrad at ten years. Simulant mixtures of ion exchange resins were irradiated within the calculated dose range. Resins were irradiated as received, i.e., as air-dried resins and as resin/water slurries. Analyses of vapor space gas contents indicate that hydrogen is the predominant radiolytic gas species produced. Hydrogen yields expressed as G values were in the range of G(H2) = 0.3 to 0.8 molecules/100 eV. The G value expresses number of molecules produced per 100 eV radiation absorption.
One purpose of these radiation experiments was to provide data applicable towards sampling, agitation and potential safety systems handling of ion exchangers by the Argentines as they prepare to immobilize the resin slurries. Several calculations involving actual analyzed resins, measured radiolytic hydrogen yields, and existing storage container geometries were performed. Storage of relatively small ~50 m3 resin slurries in large 200 m3 storage containers should not involve buildup of flammable hydrogen concentrations (4 vol% H2) in air over a 10-year period. However, as the fraction of storage volume occupied by the resin increases, calculated flammability times occur within 1 to 5 years. Soluble carbon analyses from irradiated resin slurries indicate no significant radiolytic degradation of the resin occurs over a 20 Mrad dose. Resins displayed excellent cesium, cobalt and strontium cation retention capabilities for doses approaching 70 Mrad. No significant physical effects such as agglomeration or viscosity changes were observed when irradiated resin slurries were compared to control unirradiated slurry samples.
Introduction and Background
A recent technology exchange between Argentina Nuclear Energy Commission (CNEA) and the US Department of Energy involved vitrification studies of ion exchange resins. Details of the spent ion exchange resins currently stored at two Argentine nuclear power plants, Atucha I and Embalse, have been presented in earlier reports.1,2 The present study examines irradiation of simulant samples of ion exchange resins. Activities reported involve:
The intent of these radiation experiments is to provide data that can be applied towards sampling, agitation and potential safety systems in the handling of ion exchangers by the Argentines as they prepare to immobilize the resin slurries. The resins are planned to be immobilized either as grout or vitrified wasteforms.1,2 Some of the irradiated resin slurries obtained from the present investigations were also used in related bench-scale vitrification studies performed at the Savannah River Technology Center (SRTC). Similar radiation effects studies on ion exchange resins have recently been reported from SRTC. 3,4 Several review articles have also been published that review past radiation investigations on ion exchangers. 5,6
Experimental
Resin Samples
Resin samples were prepared to simulate the Atucha I and Embalse resin mixtures. Cicero-Herman has previously reported in detail various resin mixes used to approximate the actual resins used at the Argentine nuclear facilities.2 Ion exchange material is actually stored at the Argentine nuclear facilities as aqueous slurries.1 Resin to water ratios in the resin slurries were previously determined to be representative of actual reported resin slurry ratios.1,2 Embalse resin slurry contained 0.43 g Embalse resin simulate mixture to 1 mL of deionized water. Atucha I resin slurry contained 0.75 g Atucha I resin simulate mixture to 1 mL of deionized water.
Irradiations
Gamma irradiations were performed using a Co-60 source at SRTC. Two different sources were used with dose rates of about 5E+05 rad/hr and 1.4E+06 rad/hr. Dose rates are based on dosimetry performed with thin film dosimeters containing radiometric dye.7 Temperatures during the irradiations were ~30 ° C. All irradiations were performed in 60-mL or 160-mL glass serum vials. Tests designed to measure vapor gases used 60-mL vials sealed with crimp-top caps to contain radiolytically produced gases. Relatively small 2-6 gram sample masses were irradiated to prevent significant pressure changes within the 60-mL air-sealed vials during irradiation. Immediately after removal from the radiation source, slight pressure changes, i.e., -3 to +0.5 psi, in irradiated samples were measured with a -5 to +30 psi mechanical pressure gauge fitted with a syringe needle. Irradiated sample bottles were briefly equilibrated by venting to atmospheric pressure immediately before gas analysis. Radiolytic production values, or G values, were calculated for individual gas components from the final composition of the gas, the final dose, the void volume in the system, and the ideal gas law assuming an equilibrated pressure of 1 atmosphere.
Analytical Methods
Gases produced from radiolysis of resins and resin slurries were analyzed by gas chromatography with a Varian Model 3400 gas chromatograph (thermal conductivity detector and flame ionization detector, chromosorb-101 and molecular sieve columns, argon carrier gas). Gases detected and analyzed for were H2, O2, N2, CO, CO2 and CH4. Standard gases (Scott Specialty Gases, accuracy = +/- 2%) containing these components in the range of 1-10 vol% were used for calibration. Total inorganic and organic carbon measurements were made on filtered resin/water leachates using a O.I. Co. Total Carbon analyzer. These leachates were also analyzed for soluble anions using a Dionex ion chromatography instrument (Dionex AS4/AG4 column, equimolar NaHCO3/Na2CO3 eluent, conductivity detection). Effects of radiation on resin retention of nonradioactive Cs, Co and Sr were studied by analyzing resin/water leachates containing these cations both prior to and immediately after irradiation. Aqueous samples were analyzed for Cs by atomic absorption spectroscopy (AAS) and Co/Sr were determined by inductively coupled plasma emission spectroscopy (ICP-ES).
Results
Calculated Radiation Dose from Spent Resins
A sample of stored spent resin from the Atucha I Nuclear Power Plant has been characterized by the Argentines.1 The radioisotope contents are shown in Table 1. Based on total beta/gamma specific activity and the radionuclides present in the ratios shown in Table 1, calculational techniques exist that allow estimation of the total accumulated dose to the resins as a function of time.8 Appendix A contains the detailed calculations. Figure 1 shows accumulated doses as a function of time that result from these calculations. Gamma doses were assumed to be about 75% of the total gamma energy, i.e., about 25% of the gamma energy is lost to the surroundings.8 It can be seen that most of the total beta/gamma dose results from Cs-137(beta)/Ba-137(gamma) nuclides with some contribution from the Co-60(gamma) and relatively little dose from Cs-134(beta/gamma). The total ten-year dose approaches about 4.5E+06 rad.
Table 1. Radiochemical Analytical Results for Atucha I Resin
|
Total Beta/Gamma Activity |
1.5 E+12 Bq/m3 |
|
|
|
|
Component |
Relative Amount |
|
Cs-137 |
68.6 % |
|
Co-60 |
25.3 % |
|
Cs-134 |
4.6 % |
|
Others |
|

Figure 1. Absorbed Dose Versus Time for Atucha I Resin.
Radiolytic Gas Yields
Resin samples were prepared to simulate the Atucha I and Embalse resin mixtures and were irradiated with and without water present. Cicero-Herman has previously reported in detail the various resin mixes used to approximate the actual resins used at the Argentine nuclear facilities.2 All systems were analyzed for vapor gas space contents by gas chromatography. Table 2 shows the results of these analyses. Hydrogen, Oxygen and Nitrogen were the main gas species detected with only trace < 0.1 vol% amounts of COx and CxHx present. Oxygen and Nitrogen were present in the initial air-sealed test vessels. Component concentrations in the gas were used to calculate radiolytic production terms, or G-values for hydrogen and oxygen. The G-value is expressed as # molecules produced or consumed per 100 eV of ionizing radiation absorbed. Appendix B contains a detailed calculation of G-values obtained using the ideal gas law and the experimentally determined gas component concentrations. Table 2 also shows G-values calculated for the radiolytic production of hydrogen and radiolytic consumption of oxygen.
Table 2. Gas Concentrations and Radiolytic Yields
|
Resin System |
Dose (Mrad) |
Vol.% H2, G(H2)* |
Vol.% O2, G(O2)* |
|
|
|||
|
Embalse Resin |
5.4 |
0.17, 0.35 |
16.50, -8.81 |
|
18.6 |
0.73, 0.44 |
8.91, -7.15 |
|
|
29.8 |
0.96, 0.36 |
4.10, -6.21 |
|
|
Atucha I Resin |
4.7 |
0.25, 0.62 |
18.58, -5.37 |
|
17.4 |
1.21, 0.74 |
9.43, -6.98 |
|
|
21.7 |
1.55, 0.80 |
7.21, -6.99 |
|
|
Embalse Slurry |
5.2 |
0.67, 0.40 |
19.02, -1.06 |
|
19.0 |
1.87, 0.31 |
15.26, -0.91 |
|
|
22.0 |
2.41, 0.34 |
13.27, -1.06 |
|
|
Atucha I Slurry |
5.5 |
0.77, 0.63 |
18.55, -1.84 |
|
21.1 |
3.01, 0.63 |
12.93, -1.72 |
|
|
26.0 |
3.51, 0.66 |
12.89, -1.42 |
The G(H2) values determined in these resin systems are in the range of 0.3 to 0.8. Typical G(H2) values from previously reported analogous resin irradiation studies are in the range of 0.1 to 0.6.8 Both resin systems showed higher radiolytic hydrogen production from resin/water slurries relative to irradiation of the resins only. Radiolytic hydrogen production from water is G(H2) = 0.45.9
Radiolytic oxygen depletion was more significant for the resin irradiations relative to the resin/slurry irradiations. Depletion of oxygen is widely reported in resin radiolysis experiments. The mechanism involves scission of the carbon-carbon bonds of the resin structure by direct interaction of radiation on the resin, followed by carbon-centered radical reactions with gaseous O2. Less oxygen depletion is observed in the resin/water slurries because the resins are not in contact with vapor space oxygen in the air-sealed containers. All resins in simulant mixes in these experiments on resin/water slurries were observed to sink to the bottom of the glass irradiation vessels, indicating that their density is slightly greater than the purified water.
Leachate Analyses
Leachates from resin/water slurries were analyzed for total inorganic/organic carbon (TIC/TOC) and soluble anions. Table 3 contains results from the soluble carbon analyses. No significant amount of any soluble anions above 10 mg/L concentrations were detected in any of the irradiated and unirradiated leachates. The soluble carbon data indicates that irradiation in the dose range of about 22 Mrad does not significantly degrade the resin. Soluble carbon concentrations were similar for both the unirradiated samples and all irradiated samples.
Table 3. Soluble Carbon Analyses
|
Resin System |
Dose |
Total Inorganic |
Total Organic |
|
Embalse Slurry |
22.0 |
357 |
4998 |
|
22.0 |
231 |
5607 |
|
|
0 |
273 |
6951 |
|
|
Atucha I Slurry |
21.1 |
441 |
5187 |
|
21.1 |
357 |
5103 |
|
|
0 |
273 |
5019 |
Separate irradiation tests were carried out to investigate the effects of radiation on the different resin mixes’ capability to retain the cations Cs, Co and Sr. Tests were performed in duplicate. A stock solution containing Cs, Co and Sr at about 500 mg/L concentration of each species was added to resin simulant mixes to produce resin slurry mixtures. Portions of the aqueous phase of resin/water slurries were analyzed prior to and immediately after irradiation. Table 4 contains results from these leachate analyses. Data from Table 4 for unirradiated slurries indicates that all cations present in the initial resin/water slurries were essentially completely sorbed by the resin. Irradiation caused no significant loss of the various cations by the resins.
Table 4. Leachate Cation Analyses
|
Leachate Concentrations |
||||
|
Resin System |
Dose (Mrad) |
Cs, mg/L |
Co, mg/L |
Sr, mg/L |
|
Embalse Resin |
71.1 |
0.35 |
0.007 |
0.046 |
|
Slurry |
71.1 |
0.35 |
<0.007 |
0.046 |
|
0 |
0.38 |
0.008 |
0.045 |
|
|
0 |
0.38 |
<0.007 |
0.045 |
|
|
Atucha I Resin |
71.1 |
2.85 |
1.884 |
0.145 |
|
Slurry |
71.1 |
3.07 |
13.20 |
0.080 |
|
0 |
1.38 |
0.099 |
0.053 |
|
|
0 |
1.27 |
0.089 |
0.047 |
|
Flammability Calculations
During the storage of spent ion exchange resins H2 from radiolysis can form a flammable gas mixture (4% hydrogen in air) in the vapor space above the resins if this space is not ventilated. Calculations involving radiolytic hydrogen generation in sealed containers have been reported.8 Equation #1 is used to calculate the time required to reach a given concentration of hydrogen.8
H2% = D * M * G(H2)/100 * C * (100%/V) (Eqation #1)
with:
It is evident from equation #1 that the percentage of hydrogen in sealed storage containers is directly proportional to the total dose, amount of resin and G(H2) value. Volume percent hydrogen amounts are inversely proportional to the free volume in the container.
Argentine Storage Condition Calculations
Calculational data discussed earlier for the Atucha I resins and experimentally determined G(H2) values for both the Atucha I and Embalse resin slurry systems have been used to calculate times to reach flammable hydrogen mixtures in air. Storage geometries and resin masses have been approximated from details reported by the Argentines.1 Several cases actually in use for resin storage by the Argentines were considered. In one scenario a nearly full 15 m3 storage tank is used to calculate the flame time for storage of about 14 m3 Atucha I resin slurry. In others, a 200 m3 cistern is assumed to contain all of the Atucha I resin slurry equal to 42 m3 and in a different case, is assumed to contain all of the Embalse resin slurry equal to 130 m3.
Table 5 shows the calculated hydrogen concentrations from storage of the various resin slurries from 1 to 10 years. In Table 5 the calculated doses based on actual analyses of the Atucha I resins (See Table 1 and Figure 1) are assumed to be the same for the Embalse resins since no radiochemical analyses were reported earlier for the Embalse resins.1 Radiolytic production yields of hydrogen buildup, i.e., G(H2) values, in the sealed container headspace used in Table 5 were taken from the experimentally determined yields reported in Table 2. Figure 2 shows the hydrogen production data for the various storage conditions.
Data shown in Table 5 and plotted in Figure 2 show that storage of large amounts of resin in nearly full 15 m3 sealed tanks would result in hydrogen concentrations above the flammability limit of 4 vol% in less than 1 year. A larger 200 m3 cistern containing all of the 42 m3 of Atucha I resin slurry would only reach about 1.0 vol% H2 in 10 years. Similar storage of all the 130 m3 Embalse resin slurry would produce a flammable H2 mixture in air in less than five years.
Table 5. Radiolytic Hydrogen Production From Storage of Resins
|
Resin System |
Total |
Resin Volume |
Free |
G(H2) |
Vol.% H2 |
Vol.% H2 |
Vol.% H2 |
|
Atucha I |
15 |
14 |
1 |
0.35 |
6.7 |
29.3 |
51.3 |
|
Atucha I |
200 |
42 |
158 |
0.35 |
0.2 |
0.6 |
1.0 |
|
Embalse |
200
|
130
|
70 |
0.65 |
1.7 |
7.2 |
12.6 |
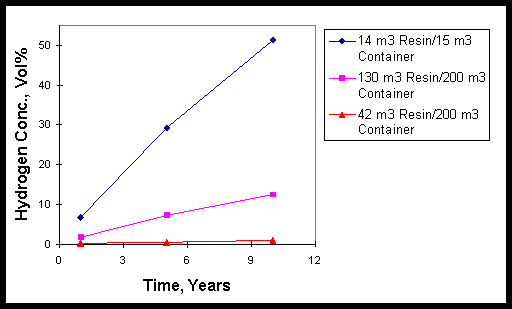
Figure 2. Hydrogen Concentration Values Plotted Versus
Time for Various Resin Slurry Storage Geometries.
Conclusions
Irradiation studies have been performed on representative simulant resins and resin slurries from Argentine Nuclear facilities. Several storage conditions were considered in attempts to predict times to reach hydrogen flammability (in air) in sealed containers in which spent resins slurries are stored. The calculations used both predicted total accumulated doses from data reported by the Argentines and experimentally determined radiolytic gas yields measured at SRTC. The calculations indicate that for relatively low amounts of resin stored in large 200 m3 containers, flammability times will exceed 10 years. However, geometries representative of relatively large resin volumes stored in either 15 m3 or 200 m3 containers indicate flammability times occur within 1 to 5 years. These data indicate that safety precautions involving such measures as vapor space ventilation to deplete hydrogen and elimination of any possible ignition sources should be considered in the safe handling of the stored resins in air-sealed containers.
These calculations are applicable to long-term storage of resins in air-sealed containers with no ventilation. Radiolytic hydrogen production from spent resins should not present significant concerns in the vitrification of these materials. Vitrification activities in glass melters are typically of much shorter duration, i.e., hours to days, and thus would not involve the time needed to buildup radiolytically generated gaseous hydrogen for flammability concerns.
Analyses of soluble carbon product formation from irradiated and unirradiated resins indicate that insignificant resin degradation is caused by absorption of about 20 Mrad dose. Radiolysis tests also indicate that these resins can retain up to 500 mg/L of various cations after absorption of about 70 Mrad dose.
In all irradiation experiments conducted for this study in the dose range of 20 to 70 Mrad, no significant changes to the physical state of the resin slurries was observed. All aqueous resin slurries were observed to separate into a lower resin layer and upper aqueous layer due to the larger density of the resins than that of pure water. The present tests suggest that no significant engineering alterations should be needed, relative to the handling of unirradiated resin slurries, in planned sampling, agitation and handling of the spent resin slurries in Argentine nuclear storage facilities. Stored resin slurries would require agitation before sampling if a well-mixed representative resin/slurry sample were required as feed material to an immobilization process.
References
Technical Review
(Per GT-QA-2-8)
M. L. Hyder, Chemical & Hydrogen Technology Section, SRTC
Appendix A
Dose Calculations from Analyzed Atucha I Spent Resin Slurry
Accumulated Doses were calculated using the equation shown below (See Absorbed Dose Calculation in Reference #8). Note that for gamma emitters it is assumed that 75% of the energy is absorbed by the resin slurry with about 25% loss to surroundings. The actual fraction of gamma energy absorbed by the resin systems depends on the resin slurry density, the geometry of the storage containers, and the energy of each gamma emitting radionuclide.8
Dose(t) = Constant x activity density x [Beta Energy + Low X-ray Energy +
(Gamma Energy x Intensity Abundance Fraction x 0.75)] x
{(1-exp(-t*l
))/l
}
with:
Appendix B
G Value Calculations from Vapor Space Gas Concentrations
The radiolytic gas component yields were calculated using the following equation (See equation 3.28 of Reference# 9):
G(x) = ((C * (Vol%(X)/100) * Vapor Volume)/Vm)/ (M/1000)
_____________________________________________
Total Dose
with: