WSRC-TR-2003-00063
Final Report on Jobin Yvon Contained Inductively Coupled
Plasma Emission Spectrometer (ICP-ES)
F.M. Pennebaker and J.C. Hart
Westinghouse Savannah River Company
Aiken, SC 29808
1.0 Summary
A new Inductively Coupled Plasma - Emission Spectrometer (ICP-ES) was recently purchased and installed in Lab B-147/151 at SRTC. The contained JY Model Ultima 170-C ICP-ES has been tested and compared to current ADS ICP-ES instrumentation. The testing has included both performance tests to evaluate instrumental ability, and the measurement of matrix standards commonly analyzed by ICP-ES at Savannah River. In developing operating procedures for this instrument, we have implemented the use of internal standards and off-peak background subtraction. Both of these techniques are recommended by EPA SW-846 ICP-ES methods and are common to current ICP-ES operations. Based on the testing and changes, the JY Model Ultima 170-C ICP-ES provides improved performance for elemental analysis of radioactive samples in the Analytical Development Section.
Keywords: ICP-ES, waste tank analysis, RCRA
2.0 Introduction
2.1 Background
Late in FY 1998, ADS received capital funding (project S-W281) to purchase a contained Inductively Coupled Plasma Emission Spectrometer (ICP-ES) for radioactive sample analysis. The new instrument replaced the existing contained ARL 3580 ICP-ES, which was 15 years old. The new instrument was built by Jobin Yvon (JY) in France and is installed in laboratory B-147. A series of performance tests detailed in the specifications were performed prior to awarding the bid. These tests included spectral resolution, dynamic range, short and long term stability, sensitivity and interference control. The tests were also performed on the completed instrumentation in France and following installation at SRTC. JY had difficulty with several of the tests and spent about 6 months at SRTC reconfiguring the instrument and completing the tests. This report will document subsequent testing performed at SRTC to confirm accurate analyses.
2.2 Instrument Description
The instrument is an Inductively Coupled Plasma Emission Spectrometer (ICP-ES) with a 1.5 kWatt 40 MHz solid-state generator contained in a radiological fume hood. The spectrometer section of the instrument consists of a polychrometer for simultaneous analysis of 30 wavelengths and a monochrometer for wavelength flexibility. The monochrometer is a high-resolution design using a 1.0-m focal length Czerny-Turner (CZ) configuration, which covers from 120 nm - 800 nm with an argon purge to remove absorbing contaminants. The monochrometer uses a 2400 g/mm grating in the second order for 120-320 nm, resulting in a (D l ) resolution of 0.005-0.006 nm, full-width at half max (FWHM) and the same grating in first order from 320-800 nm resulting in resolution of 0.010-0.012 nm. Light is then measured with a high-dynamic range detection system (HDD) photomultiplier tube (PMT), which can vary the gain to accommodate a wide variety of intensities. The polychrometer uses a Paschen-Rungen mount (Rowland Circle) design with 0.5-m focal length and a 3000 g/mm grating in first order. The polychrometer also contains a flat-field segment to detect Na, Li and K. Table A-1 (Appendix) lists some of the elements/wavelengths on the polychrometer. The two main advantages to the polychrometer are speed and thermal stability. The main disadvantages are poorer resolution and stray light rejection. Thus, the monochrometer has better sensitivity while the polychrometer is more stable.
2.3 SRTC Hood Modifications
The design of the radiological fume hood in B-147 was found to be inadequate for the needs of SRTC. The radioactive fume hood had several concerns, including inadequate viewing into the hood and a large amount of torque on the glass window for stabilization. Jerry McCarty designed a new window, sash and frame, which were subsequently built and welded together by the machine shop and a local area vendor. The new equipment has been successfully installed in lab B-147. Minor modifications were also made to the brackets and counterweights for easier sash movement.
3.0 SRTC Instrument Testing
ADS evaluated the system for sensitivity, dynamic range, short and long term stability and spectral interference. The tests were similar to the performance tests, but under typical operating conditions (i.e. MDL's were measured for polychrometer lines rather than for optimum detection limits). Testing was also performed to identify typical problems such as PMT overrange and document polychrometer peak locations. The tests are listed in Table 2, and a complete listing of the results are available in Notebook WSRC-NB-2000-120. Most elements showed good stability and reasonable sensitivity. Corrective actions were taken for those elements with poor stability or sensitivity. Several operational changes were made due to the new instrumentation. These operational changes include background correction, gain settings on PMT's, use of guassian mode of peak calculation and use of an internal standard.
3.1 Initial Documentation
Initial tests were performed to document ICP performance after installation. We measured the signal and Relative Standard Deviation (%RSD) for 10 mg/L analyte, background intensity, detection limits and polychrometer position location with centering on Cr for a variety of wavelengths. This initial documentation will provide a baseline to evaluate performance over time. This documentation should also help evaluate problems during instrument breakdown.
3.2 Spectrometer Resolution
The JY ICP performed very well on the resolution performance tests at SRTC and France. Results demonstrated the 0.005-0.006 nm resolution in second order and 0.010-0.012 nm resolution in first order. We found no need for further testing on the resolution. The results are included below.
Table 1. Performance Test 3.1.1 Resolution
|
Description |
Bid Package |
SRTC Results |
|
FWHM of Al 167 nm |
0.0044 nm |
0.0044 nm |
|
Resolution of Tl doublet at 190.84 |
0.005 nm |
0.00489 nm |
|
Fe 310.04 - 3 peaks should be resolved and 4th partially resolved |
ok |
ok |
|
As and Cd resolved at 228.802 |
resolved (D l = 0.0043) |
Resolved (D l = 0.0042) |
|
Ce and Fe resolved at 395.25 nm |
resolved (D l = 0.0068) |
Resolved (D l = 0.010) |
|
Nd and Ce resolved at 401.22 nm |
resolved (D l = 0.009) |
Resolved (D l = 0.010) |
3.3 ICP Wavelength Stability
One of our concerns was stability of the analytical signal. There were no stipulations for ICP operating conditions in the performance tests; thus, the instrument was run with slits at maximum during testing. ICP sensitivity and interference is significantly hampered by operation in this mode. Also, SRTC has temperature fluctuations, which can significantly impact ICP stability. We performed our own stability tests to obtain a true picture of ICP long term and short term stability. Our tests showed good short-term stability for performance times less than two hours. Long-term stability was very good for polychrometer elements, but very poor for monochrometer elements.
We believe that temperature instability was a major cause of poor results on several of the performance tests at France and SRTC. The result is that monochrometer wavelengths significantly move during operation of the instrument (see Figure 1). This instability can cause as much as a 50% decrease in signal after 8 hours. Temperature instability is not evident on the polychrometer, even after 9 hours of run time. We must use an alternate mode of peak calculation called the guassian mode to prevent recalibration every hour or two for monochrometer wavelengths. For this method, 9 points are collected over the spectral region and a guassian curve is drawn from the best 5 points. If the wavelength shifts, the program should still find the maximum intensity of the peak. We are hopeful that recent modifications of the ventilation ducts in B-147/151 will improve temperature instability and allow other calculation methods.
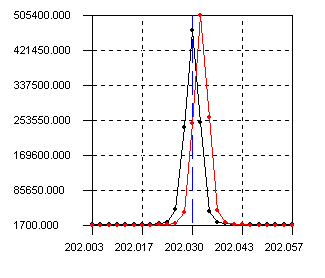
Figure 1. Change in monochrometer wavelength position
of Molybdenum 202.03 nm
from 9:00 (red) to 5:00 (black) during constant operation.
3.4 Background Interference
The existing ARL 3580 instrument had very good rejection of stray light, which has enabled SRTC to avoid using background correction until now. The JY polychrometer has approximately 30 times the stray light of the ARL. Table 2 compares the P 178.225 nm intensities on the ARL 3580 and the JY ICP-ES instruments. Some of the interference, i.e. Mo, is from direct overlap from a nearby wavelength; however, most of the increases in signal are from an increased background level caused by inadequate rejection of stray light. This increased background would cause inaccurate measurements for trace analysis. Background correction is a common practice in ICP-ES to eliminate this problem. Two points on either side of the peak are measured, averaged and subtracted from the peak. However, this is a significant change from our previous "hot" operation. The CTF over the instrument will need to be cognizant of background interference biasing numbers low. We have looked at interference on the polychrometer elements and selected the best correction positions based on common matrices and interference intensity.
Table 2. Comparison of measured concentration of ARL
3580 and JY ICP-ES instruments
for P at 178 nm. High concentrations are mostly the result of background
interference,
which can be eliminated with background subtraction.
|
Solution |
JY ICP-ES |
ARL ICP-ES |
JY with Bk Corr |
|
1P 10 mg/L |
7.90 |
10.0 |
9.12 |
|
Mn 100 mg/L |
11.4 |
0.383 |
0.161 |
|
Fe 100 mg/L |
3.24 |
0.090 |
-0.003 |
|
Mo 100 mg/L |
0.854 |
0.292 |
0.579 |
|
Zr 100 mg/L |
1.52 |
0.025 |
0.038 |
|
Ti 100 mg/L |
0.46 |
0.042 |
0.014 |
|
Sn 100 mg/L |
0.09 |
0.026 |
-0.010 |
for JY ICP indicates interference from Mn and Fe in standard.
3.5 Gain and Internal Standards
We have made other modifications to the operation of the instrument to accommodate the needs of SRTC. First, gain settings on the PMT's in the polychrometer were set at the factory for optimum detection limits rather than dynamic range. We have reset the gains in several cases to measure higher concentrations typical of ADS solutions. For example, Si could measure a maximum of 20 mg/L without PMT over-range (requiring additional dilution for successful quantitation). We have reset the gain to measure at least 100 mg/L.
Second, we have added the use of an internal standard. This change should improve analysis of high concentrations of salt. At first, we attempted to use scandium on the monochrometer as an internal standard; however, the JY software automatically shifts from the guassian mode to peak height mode. We used Ce 399.924 nm on the polychrometer as an internal standard for Comparison Testing (Section 4.0). We have added a Scandium channel to the polychrometer since that time for internal standard correction.
4.0 SRTC Comparison ICP-ES Testing
Common analytical samples were analyzed on the current SRTC ICP-ES systems, "cold" ICP-ES (Optima 3000) and "hot" ICP-ES (ARL 3580), and compared to the JY. All samples and standards were non-radioactive and represent the wide range of samples analyzed by ADS. The results are split into common samples (Section 4.1) from selected ADS customers and solid and liquid standards (Section 4.2) from various labs.
4.1 ICP-ES Comparison of Common Samples
We selected two sets of samples for analysis by the JY: 300170118-300170129 and 300166455-300166465. These samples were selected because they had a relatively small number of elements and were representative of the high salt samples typically analyzed by ADS. The samples were run at 1000-fold dilution to measure sodium and 100-fold dilution to measure aluminum and silicon. The results for samples 170118-170129 are compiled below in Tables 3 and 4.
Table 3. Comparison of sodium and silicon analysis
with both JY and Optima
Instruments. Concentrations are measured in units of mg/L.
|
Sodium Analysis |
Silicon Analysis |
|||||
|
Sample ID |
JY |
Optima |
% Difference |
JY |
Optima |
% Difference |
|
170118 |
77000 |
80700 |
-4.7 |
116 |
97 |
18 |
|
170119 |
77200 |
80600 |
-4.3 |
128 |
107 |
18 |
|
170120 |
78200 |
81000 |
-3.5 |
214 |
193 |
10 |
|
170121 |
78000 |
80100 |
-2.7 |
217 |
201 |
7.5 |
|
170122 |
77800 |
81900 |
-5.1 |
401 |
376 |
6.4 |
|
170123 |
78700 |
82400 |
-4.6 |
405 |
384 |
5.4 |
|
170124 |
81700 |
84500 |
-3.4 |
119 |
108 |
9.2 |
|
170125 |
81800 |
84900 |
-3.7 |
112 |
105 |
6.6 |
|
170126 |
82700 |
86500 |
-4.5 |
229 |
219 |
4.2 |
|
170127 |
81100 |
82700 |
-2.0 |
205 |
197 |
4.2 |
|
170128 |
82000 |
86800 |
-5.7 |
346 |
330 |
4.7 |
|
170129 |
82000 |
86300 |
-5.1 |
321 |
319 |
0.8 |
Table 4. Comparison of aluminum analysis with JY and
Optima ICP-ES Instruments.
Concentrations are measured in units of mg/L.
|
Al Analysis |
|||
|
Sample ID |
JY |
Optima |
% Difference |
|
170118 |
3410 |
3330 |
2.4 |
|
170119 |
3490 |
3440 |
1.4 |
|
170120 |
3220 |
3190 |
0.9 |
|
170121 |
3350 |
3320 |
0.9 |
|
170122 |
3260 |
3280 |
-0.6 |
|
170123 |
3140 |
3110 |
1.0 |
|
170124 |
4830 |
4790 |
0.8 |
|
170125 |
4970 |
4930 |
0.8 |
|
170126 |
4420 |
4400 |
0.5 |
|
170127 |
4290 |
4290 |
0.0 |
|
170128 |
4780 |
4700 |
1.7 |
|
170129 |
4360 |
4380 |
-0.5 |
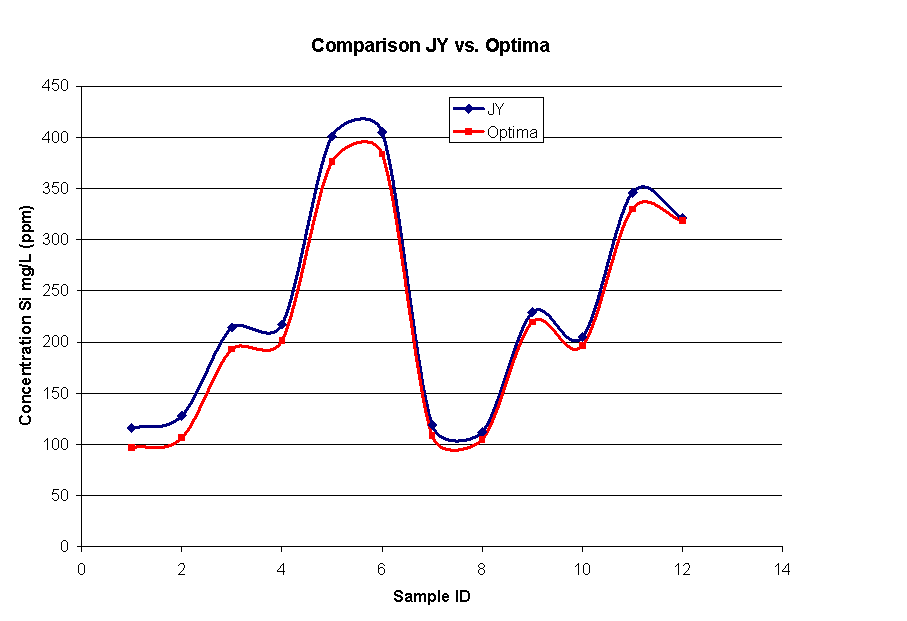
Figure 2. Comparison of Si measurements in samples
170118-170129
from Tank 38 Si removal studies.
For these samples, the two instruments compared well for Al and Na measurements. Theinstruments also compared well for Si except for the first three samples, which contain low levels of Si in a high sodium matrix. A high bias for low levels of Si was found for the JY instrument and is discussed in ADS document SRT-ADS-2002-0343. Figure YY shows a comparison plot of Si concentration in each sample.
A comparison of samples 166455-166465 was also performed by the two ICP-ES systems. The results are shown in Tables 5 and 6 below.
Table 5. Comparison of sodium and aluminum analysis
with both JY and
Optima Instruments. Concentrations are measured in units of mg/L.
|
Sodium Analysis |
Aluminum Analysis |
|||||
|
Sample ID |
Optima |
JY |
% Difference |
Optima |
JY |
% Difference |
|
166455 |
160000 |
158000 |
-1.3 |
3440 |
3350 |
-2.8 |
|
166456 |
136000 |
135000 |
-0.7 |
37.0 |
31.0 |
-18.3 |
|
166457 |
134000 |
137000 |
2.2 |
222 |
207 |
-6.6 |
|
166458 |
137000 |
137000 |
0.0 |
452 |
436 |
-3.7 |
|
166459 |
137000 |
139000 |
1.5 |
634 |
607 |
-4.3 |
|
166460 |
150000 |
144000 |
-4.1 |
986 |
944 |
-4.4 |
|
166461 |
150000 |
150000 |
0.0 |
1480 |
1410 |
-4.9 |
|
166462 |
155000 |
157000 |
1.3 |
1980 |
1880 |
-5.3 |
|
166463 |
168000 |
161000 |
-4.3 |
2210 |
2070 |
-6.6 |
|
166464 |
163000 |
165000 |
1.2 |
2130 |
2020 |
-5.5 |
|
166465 |
170000 |
166000 |
-2.4 |
2060 |
1940 |
-5.7 |
Table 6. Comparison of silicon analysis with JY and
Optima ICP-ES Instruments.
Concentrations are measured in units of mg/L.
|
Si Analysis |
|||
|
Sample ID |
Optima |
JY |
% Difference |
|
166455 |
2980 |
2970 |
-0.4 |
|
166456 |
80.3 |
79.4 |
-1.1 |
|
166457 |
230 |
222 |
-3.6 |
|
166458 |
437 |
413 |
-5.5 |
|
166459 |
597 |
561 |
-6.1 |
|
166460 |
886 |
850 |
-4.2 |
|
166461 |
1290 |
1220 |
-5.3 |
|
166462 |
1660 |
1570 |
-5.4 |
|
166463 |
1810 |
1690 |
-6.9 |
|
166464 |
1680 |
1580 |
-6.4 |
|
166465 |
1570 |
1470 |
-6.8 |
These samples had a much higher variation in Si and Al concentration, but the measurements for these elements were very good with the JY instrument. In all cases, except for Al in sample 166456, the results were within 7% of the concentration found by the current ADS "non-radioactive" ICP-ES. In the single anomalous case, Al concentration after dilution was very low, and the difference is within the expected error for such a low measurement.
4.2 ICP-ES Comparison of Common Standards
While the results of section 4.1 demonstrated the consistency in measurement between the two instruments, accuracy can only be demonstrated on standard materials with known concentrations. Nine different solid and liquid standards traceable to NIST were prepared and analyzed by all three ADS ICP-ES instruments for comparison. The results are shown in Tables 7-15 below. The results demonstrate very good accuracy for the elements measured. There are a few exceptions to note. First, Aluminum measured low for the BCR sludge standard for all three instruments, most likely due to incomplete dissolution. Second, analyses of trace components (i.e. Cd) in solids and drinking water were difficult due to the low concentration in the final solution. Lastly, line selection on the polychrometer played a significant role in the analysis. Trace levels of barium consistly measured high on JY and ARL ICP’s due to the use of different wavelength from the Optima instrument. Overall, the instrument performed very well on these standards and should improve, as the operators become more familiar with the instrument and its operation.
Table 7. Elemental analysis of LOAM-B soil sample digested
by fusion and microwave
ADS followed by ICP-ES analysis. Concentrations are measured in units
of ug/g.
|
LOAM B – soil sample ADS 165864 (Concentration in ug/g) |
|||||
|
Element |
JY |
Optima |
ARL |
Expected |
% Difference (JY) |
|
Al |
51000 |
49800 |
51600 |
51400 |
-0.8 |
|
Ca |
3400 |
3470 |
3420 |
3390 |
0.3 |
|
Fe |
27700 |
27600 |
29200 |
27400 |
1.1 |
|
Mg |
3970 |
3730 |
4190 |
4040 |
-1.7 |
|
Mn |
1570 |
1520 |
1670 |
1590 |
-1.3 |
|
Na |
4630 |
4250 |
4710 |
4110 |
12.7 |
|
Si |
363000 |
346000 |
352000 |
NA |
NA |
|
Sr |
146 |
166 |
174 |
142 |
2.8 |
|
Ti |
7700 |
7640 |
7630 |
(5700) |
NA |
|
Cd |
116 |
93 |
105 |
92 |
26.1 |
Table 8. Elemental analysis of ARG glass standard by
fusion and microwave digestions
followed by ICP-ES analysis. Concentrations are measured in units of ug/g.
|
ARG Glass Std - ADS 165865 (Concentration in ug/g) |
|||||
|
Element |
JY |
Optima |
ARL |
Expected |
% Difference (JY) |
|
Al |
24500 |
24600 |
24800 |
25000 |
-2.0 |
|
B |
24300 |
26400 |
NA |
26900 |
-9.7 |
|
Ba |
983 |
803 |
969 |
790 |
24.4 |
|
Ca |
10100 |
10300 |
10800 |
10200 |
-1.0 |
|
Cr |
723 |
669 |
811 |
640 |
12.9 |
|
Fe |
99700 |
98000 |
103000 |
97900 |
1.8 |
|
Li |
15200 |
14800 |
15800 |
14900 |
2.0 |
|
Mg |
5220 |
4800 |
5540 |
5200 |
0.4 |
|
Mn |
14900 |
14200 |
15400 |
14600 |
1.7 |
|
Na |
89500 |
84500 |
89100 |
85200 |
5.0 |
|
Ni |
8270 |
8020 |
8250 |
8270 |
6.9 |
|
Si |
227000 |
222000 |
240000 |
224000 |
1.3 |
|
Ti |
7110 |
6900 |
7150 |
6900 |
3.0 |
Table 9. Elemental analysis of LRM glass standard by
fusion and microwave digestions
followed by ICP-ES analysis. Concentrations are measured in units of ug/g.
|
LRM Glass Std - ADS 165866 (Concentration in ug/g) |
|||||
|
Element |
JY |
Optima |
ARL |
Expected |
% Difference (JY) |
|
Al |
51600 |
50800 |
52700 |
50600 |
2.0 |
|
B |
22400 |
24700 |
25400 |
24800 |
-9.0 |
|
Ca |
3900 |
3920 |
3690 |
3830 |
1.8 |
|
Cd |
1450 |
1420 |
1450 |
1380 |
5.1 |
|
Cr |
1470 |
1360 |
1390 |
1310 |
11.9 |
|
Fe |
10300 |
10300 |
10400 |
9940 |
4.2 |
|
Li |
522 |
512 |
485 |
497 |
4.9 |
|
Mg |
660 |
545 |
648 |
615 |
7.3 |
|
Mn |
601 |
579 |
595 |
608 |
-1.2 |
|
Na |
161000 |
156000 |
163000 |
153000 |
5.6 |
|
Ni |
1610 |
1500 |
1360 |
1460 |
10.2 |
|
Pb |
815 |
904 |
672 |
891 |
-8.6 |
|
Si |
263000 |
241000 |
260000 |
257000 |
2.3 |
|
Ti |
657 |
622 |
607 |
629 |
4.3 |
|
Zr |
7710 |
7100 |
7290 |
6740 |
14.4 |
Table 10. Elemental analysis of BCR Sludge standard
by fusion and microwave digestions
followed by ICP-ES analysis. Concentrations are measured in units of ug/g.
|
BCR Sludge Standard - ADS 165867 (Concentration in ug/g) |
|||||
|
Element |
JY |
Optima |
ARL |
Expected |
% Difference (JY) |
|
Al |
25900 |
24600 |
27600 |
47600 |
-45.6 |
|
Ca |
92600 |
95600 |
98800 |
101000 |
-8.8 |
|
Cd |
106 |
81 |
85 |
78 |
36.4 |
|
Fe |
19400 |
19100 |
20300 |
18500 |
4.7 |
|
Mg |
17000 |
15900 |
18000 |
19900 |
-14.6 |
|
Mn |
608 |
571 |
633 |
588 |
3.4 |
|
Na |
2190 |
2410 |
2220 |
2230 |
-1.6 |
|
Ni |
330 |
316 |
246 |
280 |
17.9 |
|
P |
28900 |
28900 |
31000 |
25700 |
12.2 |
|
Pb |
1430 |
1230 |
1200 |
1270 |
12.6 |
|
Si |
107000 |
103000 |
124000 |
107000 |
0.4 |
|
Ti |
17100 |
16900 |
18000 |
17400 |
-2.0 |
|
Zn |
4230 |
4080 |
3850 |
4060 |
4.2 |
Table 11. Elemental analysis of SRS Frit 202 by fusion
and microwave digestions
followed by ICP-ES analysis. Concentrations are measured in units of ug/g.
|
Frit 202 - ADS 165868 (Concentration in ug/g) |
|||||
|
Element |
JY |
Optima |
ARL |
Expected |
% Difference (JY) |
|
Al |
1490 |
1920 |
1070 |
1800 |
-17.2 |
|
B |
22800 |
24900 |
26900 |
24500 |
-6.9 |
|
Ca |
2780 |
2870 |
2870 |
NA |
NA |
|
Fe |
308 |
338 |
301 |
266 |
15.9 |
|
Li |
31600 |
30300 |
32500 |
32100 |
-1.6 |
|
Mg |
12500 |
11900 |
13100 |
11900 |
4.9 |
|
Na |
46000 |
45000 |
47200 |
43900 |
4.8 |
|
Si |
368000 |
356000 |
362000 |
356000 |
3.4 |
|
Ti |
1650 |
1630 |
1640 |
NA |
NA |
Table 12. Elemental analysis of Drinking Water Standard
from High Purity Standards
by ICP-ES analysis. Concentrations are measured in units of mg/L.
|
Drinking Water Standard - ADS 165824 (Concentration in mg/L) |
|||||
|
Element |
JY |
Optima |
ARL |
Expected |
% Difference (JY) |
|
Al |
0.120 |
0.132 |
0.123 |
0.120 |
-0.1 |
|
Ba |
0.044 |
0.051 |
0.050 |
0.050 |
-12.7 |
|
Ca |
31.2 |
34.9 |
36.5 |
35.0 |
-10.8 |
|
Cd |
0.007 |
0.011 |
0.013 |
0.010 |
-30.0 |
|
Cr |
0.019 |
0.021 |
0.041 |
0.020 |
-5.0 |
|
Cu |
0.018 |
0.024 |
0.020 |
0.020 |
-9.5 |
|
Fe |
0.094 |
0.099 |
0.104 |
0.100 |
-6.0 |
|
Mg |
8.81 |
9.22 |
8.99 |
9.00 |
-2.1 |
|
Mn |
0.038 |
0.040 |
0.040 |
0.040 |
-5.0 |
|
Na |
5.98 |
6.42 |
6.09 |
6.00 |
-0.3 |
|
Ni |
0.060 |
0.060 |
0.062 |
0.060 |
0.0 |
|
P |
<0.012 |
<0.076 |
<0.035 |
NA |
NA |
|
Pb |
<0.066 |
<0.077 |
0.070 |
0.040 |
NA |
|
Si |
<0.008 |
<0.014 |
0.114 |
NA |
NA |
|
Sr |
0.24 |
0.26 |
0.25 |
0.25 |
-5.2 |
|
Ti |
0.001 |
<0.016 |
<0.001 |
NA |
NA |
|
Zn |
0.066 |
0.077 |
0.069 |
0.070 |
-5.7 |
Table 13. Elemental analysis of Hanford Envelope A
Simulant by ICP-ES analysis.
Concentrations are measured in units of mg/L. No standard values available.
|
Envelope A Simulant - ADS 165825 (Concentration in mg/L) |
|||
|
Element |
JY |
Optima |
ARL |
|
Al |
1060 |
954 |
1260 |
|
B |
2.09 |
2.49 |
3.30 |
|
Ba |
<0.08 |
<0.12 |
<0.03 |
|
Ca |
3.01 |
3.04 |
3.22 |
|
Cd |
<0.06 |
<0.14 |
0.132 |
|
Cr |
57.6 |
57.6 |
68.7 |
|
Cu |
<0.40 |
<0.50 |
0.058 |
|
Fe |
<0.04 |
<0.44 |
0.133 |
|
Mg |
<0.2 |
<0.84 |
0.59 |
|
Mn |
<0.007 |
<0.09 |
0.032 |
|
Na |
11300 |
10700 |
11000 |
|
Ni |
<0.32 |
<0.62 |
0.15 |
|
P |
19.1 |
19.6 |
21.1 |
|
Pb |
<5.9 |
<6.9 |
2.69 |
|
Si |
6.8 |
16.7 |
10.5 |
|
Sr |
<0.004 |
<0.020 |
0.021 |
|
Ti |
0.050 |
<1.4 |
0.266 |
|
V |
NA |
<1.3 |
0.090 |
|
Zn |
<0.30 |
<3.7 |
0.45 |
|
Zr |
<0.050 |
<0.48 |
0.062 |
|
Ag |
NA |
<3.0 |
0.080 |
Table 14. Elemental analysis of SPEX Multi-Element
Standard by ICP-ES analysis.
Concentrations are measured in units of mg/L.
|
SPEX Multi-element Standard - ADS 165826 (Concentration in mg/L) |
|||||
|
Element |
JY |
Optima |
ARL |
Expected |
% Difference (JY) |
|
B |
494 |
535 |
532 |
500 |
-1.2 |
|
Li |
483 |
493 |
519 |
500 |
-3.4 |
|
Mg |
482 |
496 |
543 |
500 |
-3.6 |
|
Na |
508 |
517 |
530 |
500 |
1.6 |
|
P |
498 |
502 |
530 |
500 |
-0.4 |
|
Si |
476 |
459 |
558 |
500 |
-4.8 |
|
Ti |
479 |
510 |
529 |
500 |
-4.2 |
Table 15. Elemental analysis of In-house Standard by
ICP-ES analysis.
Concentrations are measured in units of mg/L.
|
ADS Standard 1 - ADS 165827 (Concentration in mg/L) |
|||||
|
Element |
JY |
Optima |
ARL |
Expected |
% Difference (JY) |
|
Al |
501 |
490 |
586 |
500 |
0.20 |
|
Na |
2360 |
2200 |
2470 |
2200 |
7.3 |
|
Si |
208 |
208 |
242 |
200 |
4.0 |
|
Pb |
9.55 |
9.87 |
11.9 |
10.0 |
-4.5 |
|
Ag |
NA |
9.67 |
14.3 |
10.0 |
NA |
|
Ba |
9.89 |
10.0 |
11.2 |
10.0 |
-1.1 |
|
Cd |
9.80 |
10.0 |
11.2 |
10.0 |
-2.0 |
|
Cr |
9.58 |
9.82 |
12.0 |
10.0 |
-4.2 |
|
Be |
NA |
9.66 |
NA |
10.0 |
NA |
5.0 Data Export and Workup
Data work-up for the JY ICP-ES was accomplished using a new Excel Macro (designed by ADS) called "JY_NewMacro_ves1" and Excel computational workbooks called "JY Template Rev 1.xls" and "JY Template Solids Rev 1.xls." The macro is documented in Notebook WSRC-NB-2001-120 pp. 56-64. For routine operations of the instrument, an excel template "JY _Liquid_Template_U_Poly.xls" is used in tandem with "JY_MDL_Master.xls." The spreadsheets have been designed by ADS and confirmed with ICP-ES data that has been calculated by hand.
6.0 Conclusions
While installation of the instrument was time-consuming for the vendor and ADS, the overall result was a reliable instrument for routine measurements of radioactive samples at SRTC. In developing operating procedures for this instrument, we have implemented the use of internal standards and off-peak background subtraction. Both of these techniques increased the accuracy of results from the ICP-ES for samples with difficult matrices. Improved accuracy has been demonstrated by the performance tests described in section 4.0 of this document. Overall, the instrument performed very well on these standards and should improve, as the operators become more familiar with the instrument and its operation.
Appendix
These tables are included to record performance and set parameters at the beginning of operation.
Table A-1. Background correction positions for polychrometer wavelengths.
|
Element |
Wavlength (nm) |
Left Background (nm) |
Right Background (nm) |
|
Al |
308.215 |
0.0127 |
0.0199 |
|
B |
208.959 |
0.0199 |
0.0199 |
|
Cd |
226.502 |
0.0127 |
0.0199 |
|
Ce |
399.924 |
0.0199 |
0.0199 |
|
Cr |
205.599 |
0.0199 |
0.0199 |
|
Cu |
224.700 |
0.0199 |
0.0199 |
|
Fe |
259.940 |
0.0127 |
0.0199 |
|
K |
766.490 |
0.0199 |
0.0199 |
|
Mn |
257.610 |
0.0199 |
0.0199 |
|
Na |
589.582 |
0.0199 |
0.0199 |
|
Ni |
216.552 |
0.0127 |
-------- |
|
P |
178.225 |
0.0199 |
0.0199 |
|
Pb |
220.353 |
0.0199 |
0.0199 |
|
Si |
251.611 |
0.0127 |
0.0199 |
|
Zn |
213.856 |
0.0199 |
0.0199 |
Table A-2. Detection limits for selected JY wavelengths
using Guassian mode
for monochrometer wavelengths.
|
Element |
Wavlength (nm) |
Poly/Mono |
MDL (mg/L) |
|
Al |
308.215 |
Poly |
0.093 |
|
B |
208.959 |
Poly |
0.013 |
|
Ba |
230.424 |
Poly |
0.008 |
|
Be |
313.042 |
Mono |
0.002 |
|
Ca |
393.367 |
Poly |
0.001 |
|
Cd |
226.502 |
Poly |
0.006 |
|
Cr |
205.599 |
Poly |
0.012 |
|
Cu |
224.700 |
Poly |
0.005 |
|
Fe |
259.940 |
Poly |
0.004 |
|
K |
766.490 |
Poly |
1.16 |
|
La |
408.672 |
Poly |
0.001 |
|
Li |
670.784 |
Poly |
0.005 |
|
Mg |
279.553 |
Poly |
0.0004 |
|
Mn |
257.610 |
Poly |
0.0007 |
|
Na |
589.582 |
Poly |
0.022 |
|
Ni |
216.552 |
Poly |
0.032 |
|
P |
178.225 |
Poly |
0.111 |
|
Pb |
220.353 |
Poly |
0.038 |
|
S |
181.978 |
Mono |
0.024 |
|
Sb |
217.581 |
Mono |
0.009 |
|
Si |
212.412 |
Mono |
0.031 |
|
Si |
251.611 |
Poly |
0.055 |
|
Si |
288.158 |
Mono |
0.041 |
|
Sn |
189.989 |
Mono |
0.1 |
|
Sr |
407.771 |
Poly |
0.0004 |
|
Ti |
334.940 |
Poly |
0.0012 |
|
V |
292.402 |
Mono |
0.002 |
|
Zn |
213.856 |
Poly |
0.003 |
|
Zr |
349.621 |
Poly |
0.005 |
|
Zr |
343.823 |
Mono |
0.010 |
|
Ce |
413.765 |
Mono |
0.094 |
|
Ce |
413.380 |
Mono |
0.097 |
|
Eu |
381.965 |
Mono |
0.0006 |
|
La |
398.852 |
Mono |
0.003 |
|
La |
379.478 |
Mono |
0.0018 |
|
Nd |
406.109 |
Mono |
0.010 |
|
Nd |
401.225 |
Mono |
0.003 |
|
Pr |
414.311 |
Mono |
0.012 |
|
Sm |
359.262 |
Mono |
0.006 |
|
Br1 |
76.557 |
Mono |
0.630 |
|
Cl1 |
67.362 |
Mono |
1.100 |
|
Hg |
253.652 |
Mono |
0.008 |
|
U |
385.958 |
Poly |
1.12 |
1 Cl and Br wavelengths are 1/2 of true wavelengths.
Table A-3. Optimized gain settings for the JY ICP-ES instrument.
|
Element |
Channel |
Factory Gain (on) |
Factory Gain (off) |
New Gain (on) |
New Gain (off) |
|
Ba |
11 |
6,5,4,3,2 |
1 |
6,5,3,1 |
4,2 |
|
Ca |
22 |
6 |
5,4,3,2,1 |
4 |
6,5,3,2,1 |
|
Cd |
10 |
6,5,4,3 |
2,1 |
6,5,4 |
3,2,1 |
|
La |
25 |
6,4,3,2 |
5,1 |
6,4,3,2 |
5,1 |
|
Li |
27 |
6,5,4 |
3,2,1 |
6,5,3 |
1,2,4 |
|
Mg |
15 |
6 |
5,4,3,2,1 |
3,4 |
6,5,2,1 |
|
Si |
12 |
6,5,4,3,2 |
1 |
6,4,2 |
5,3,1 |
|
Sr |
24 |
6,4,2,1 |
3,5 |
4,3 |
6,5,2,1 |
|
Zn |
6 |
6,5,4,2 |
1,2 |
6,4,3 |
5,1,2 |
|
Zr |
20 |
6,5,4 |
3,2,1 |
3,4 |
6,5,1,2 |