WSRC-TR-2002-00336
Caustic-Side Solvent Extraction Batch Distribution Measurements
for
SRS High Level Waste Samples and Dissolved Saltcake
W. R. Wilmarth, D. P. Healy, D. J. Wheeler, J. T. Mills,
V. H. Dukes, and D. P. DiPrete
Westinghouse Savannah River Company
Aiken, SC 29808
L. H. Delmau
Oak Ridge National Laboratory
Oak Ridge, TN
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from:
U.S. Department of Commerce, National Technical Information Service, 5285
Port Royal Road, Springfield, VA 22161,
phone: (800) 553-6847,
fax: (703) 605-6900,
email: orders@ntis.fedworld.gov
online ordering: http://www.ntis.gov/help/index.asp
Available electronically at http://www.osti.gov/bridge
Available for a processing fee to U.S. Department
of Energy and its contractors, in paper, from:
U.S. Department of Energy, Office of Scientific and Technical Information, P.O.
Box 62, Oak Ridge, TN 37831-0062,
phone: (865 ) 576-8401,
fax: (865) 576-5728,
email: reports@adonis.osti.gov
Key Words: Solvent Extraction, Cesium Removal, In-Cell Gamma Count
Summary
In 2001, the first measurements of cesium distribution behavior in actual waste samples during the Caustic-Side Solvent Extraction (CSSX) process were reported. These measurements showed acceptable behavior across the extraction, scrubbing and stripping stages of the CSSX process. However, extraction performance was not consistent with developed thermodynamic models. Therefore, additional batch tests were performed to measure the distribution coefficients with samples from F- and H-Area High level waste Tanks. The results of these tests provide the following conclusions.
Introduction
High level wastes stored at the Savannah River Site (SRS) consist of insoluble metal hydroxides/oxy hydroxides (sludge) that were formed upon neutralization of acidic PUREX canyon wastes. Additionally concentrated supernate and crystallized saltcake, formed upon evaporation of the neutralized liquid waste, are stored in million gallon storage tanks. The Defense Waste Processing Facility (DWPF) is currently vitrifying the sludge component of the high level waste.
The supernate and saltcake streams required both cesium and actinide decontamination prior to disposal at the Saltstone Production Facility. The required decontamination factors for cesium can approach 40,000. For this reason, a caustic-side solvent extraction (CSSX) process was developed by researchers at Oak Ridge National Laboratory. This cesium removal technology utilizes a 4 component solvent mixture comprised of a calixerene crown ether extractant (BOBCalixC6), an alcohol modifier (Cs-7SB), and an inhibitor (trioctylamine) dissolved in Isopar® L diluent.
The CSSX technology for cesium removal has received extensive study over the past several years. Included in this body of work has been several simulant studies, chemical and radiolytic studies, of the solvent stability, and a demonstration of the CSSX process on a 100 L sample of actual SRS High level waste from tanks 44H and 37F. The CSSX process was robust and successfully demonstrated cesium decontamination factors greater than 106.
Testing experience with actual high level waste samples is limited. The initial batch testing of actual tank supernate samples for the extraction, scrubbing and stripping of cesium showed acceptable cesium behavior as compared to the proposed facility’s flowsheet. However, this study suffered from poor cesium accountability related to possible dilution matrix effects. These dilutions were required to remove samples from the radiological shielded cells prior to gamma counting. Furthermore, Walker examined the CSSX process for utility in removing cesium from dissolved saltcake samples from Tanks 38H and 46F. Difficulty was again experienced with initial cesium accountability, but good/cesium accountability was eventually obtained and satisfactory CSSX performance was observed.
The purpose of this study was to expand the CSSX performance database for actual tank wastes and to evaluate the adequacy of the newly optimized solvent composition. Additionally, CSSX performance models, have been under development at ORNL. The study compares actual CSSX performance with samples of supernate and dissolved saltcake to the CSSX prediction models.
Experimental Details
Chemical Characterization of Tank Waste Samples
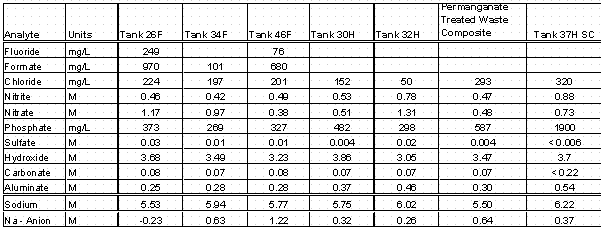
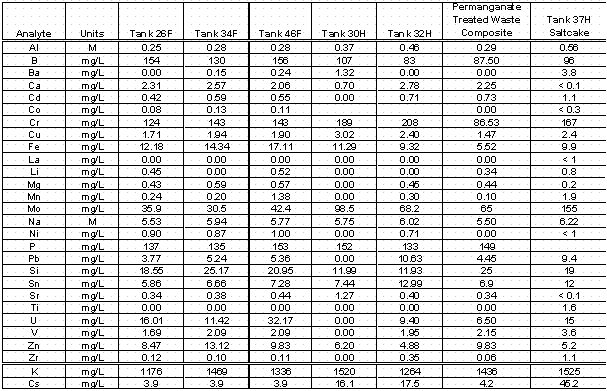
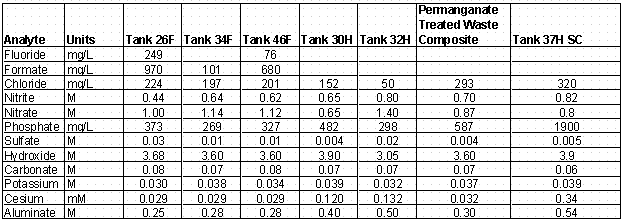
High level waste samples were obtained from several tanks in both the F- and H-Area tank farms. Previously, the 2F Evaporator system tanks (Tank 26F and 46F) were tested through the ESS protocol with only limited success. Therefore, additional samples were obtained from the feed tank (Tank 26F, samples labeled FTF-099 and FTF-100) and the drop tank (Tank 46F, samples labeled FTF-103 and FTF-104). The final samples from an F-Area tank were obtained from Tank 34F (FTF-109, FTF-110, and FTF-111). This tank represents a long-term inactive tank since no additions had been made to this tank for over 10 years. Additionally, samples were also obtained from on-going research, involving the 3H evaporator and samples from the feed tank (Tank 32H) and drop tank (Tank 30H) were allocated to CSSX ESS testing. The samples were composited by tank and gravimetrically diluted by weight with 0.2 M nitric acid. Diluted samples were removed from the cell and analyzed by Inductively Coupled Plasma-Emission Spectroscopy (ICP-ES) for sodium ion concentration. Subsequently, an aliquot of 1.6 M sodium hydroxide solution was added to each tank composite to reduce the sodium ion concentration to a target value of 5.6 M. The entire tank composite adjusted to 5.6M sodium ion concentration was then passed through a 0.45 m m filter and samples were gravimetrically diluted in dilute nitric acid for elemental concentrations and radionuclide content and by weight with water for anion analysis. Table 1 shows the analytes measured in the tank waste samples after dilution to 5.6 M sodium ion concentration.
The final two samples were obtained from other programs supporting the Salt Waste Processing Facility. The first of these was a sample from the filtrate of a Cells Unit Filter (CUF) test involving the addition of sodium permanganate to remove transuranic species. This filtrate was tested without additional dilution; however, it was passed through a 0.45 m m filter. Additionally, a sample of the Tank 37H dissolved saltcake was obtained prior to the centrifugal contactor run and was tested without alteration or sampling because it had already been filtered and characterized. This report contains characterization data for these waste samples and references to their origin.
Extraction, Scrubbing and Stripping Protocol
The ESS protocol was slightly adapted from that previously performed on the actual tank waste samples. Walker altered the protocol to include a second scrub step and this second scrub was included in the current step regiment. However, during the testing with samples of dissolved saltcake, Walker found that the centrifuge currently installed increased the temperature by ~ 5°C and could affect the measurement. Therefore, several changes were made to the ESS protocol to ensure accurate measurement in the SRTC Shielded Cells. First, the sample size of actual waste was increased to 90 ml and separatory funnels replaced the centrifuge tubes used previously. Campbell and Spence had previous success using this methodology. The standard organic-to-aqueous ratios were used and were 0.33 for the extraction and 5 for the scrubbing and stripping. Phase separation was accomplished by allowing the mixture to stand for 24 hours. The second change was to utilize the new optimized solvent composition shown in Table 2.
Table 1. Analyte Measurements for Tank Waste Samples


Table 2. Optimized Solvent Composition
|
Extractant |
BOBCalixC6 |
0.007 M |
|
Modifier |
Cs-7SB |
0.75 M |
|
Inhibitor |
Trioctylamine |
0.003 M |
|
Diluent |
IsoparÒ L |
The last change was to avoid dilution issues and count each phase neat. Therefore, the In-Cell Gamma Monitor (IGM) was used. The IGM is a 9" by 5" by 6" tungsten block into which a miniaturized NaI gamma detector has been inserted. The detector views samples in one of three positions and was designed to measure Cs-137 activities over a dynamic range of 105 to 1010 dpm/ml in the high background environment of the SRTC Shielded Cells. The initial position is in close contact with the sample container, further positions are viewed through a 0.25 inch diameter collimator. The block was coated with a plastic layer, and each sample port was designed with numerous layers of containers in the event decontamination became necessary. The gamma spectra are acquired using a Canberra Genie2k Gamma Acquisition System. Peak energy calibration was performed prior to commencement of testing and intensity calibration was performed during testing. An aliquot of 3 mL was pipetted to the counting tube and counted for 300 s. If the dead time was higher than 5%, an alternate sample position is selected. Occasionally, there was insufficient sample to obtain 3 mL for counting. In those instances the liquid phase was gravimetrically diluted to 3 mL with the fresh liquid phase. Figure 1 shows photographs of the In-Cell Gamma Monitor. The distribution coefficients were calculated based on the activity in the organic phase divided by the activity in the aqueous phase. Temperature corrections were performed using enthalpy values previously reported. All distribution coefficients are reported as corrected to 25°C.
Results and Discussion
ESS Behavior of 2F Evaporator Samples and Tank 34F
During testing in FY01, samples from the 2F Evaporator system were examined for their behavior in the ESS batch protocol. The batch equilibrium distribution coefficient, DCs, for the waste from Tank 26F measured 7.7 ± 1.9 while the measured values for Tank 46F was 13.8 ± 2.8. These values for extraction coefficients had a much larger difference than the ORNL predicted extraction coefficients that were 14.95 and 16.55 for Tank 26F and Tank 46F, respectively. In addition, the averaged extraction coefficient for Tank 26F was below the facility flowsheet criteria of a DCs of 8. The scrubbing and strip performance in the previous testing was acceptable but had high cesium uncertainties.
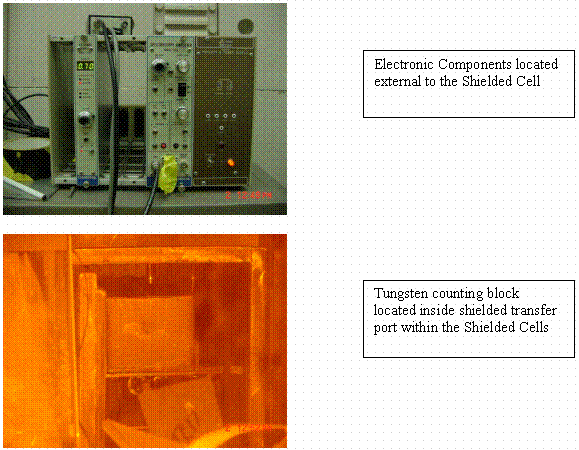
Figure 1. Photographs of In-Cell Gamma Monitor
In testing with the current samples in the shielded cells and utilizing the In-Cell Gamma Monitor, the data for the cesium behavior during the ESS testing are shown in Table 3 for the wastes collected from F-Area tanks (Tanks 26F, 46F and 34F). The extraction DCs’s for the tanks were 12.7 ± 0.14, 12.4 ± 0.57, and 11.3 ± 0.14 for Tank 26F (evaporator feed tank), Tank 46F (evaporator drop tank), and Tank 34F (concentrate storage). Additionally, the cesium accountability was much improved and was within 10%.
Table 3. ESS Batch Distribution Data for the F-Area Waste
Samples at 25° C
|
Tank 26F |
Dcs |
Std Dev |
Cs Recovery |
Std Dev |
|
Extraction |
12.7 |
0.14 |
105% |
16 |
|
Scrub 1 |
1.83 |
0.33 |
101% |
0.5 |
|
Scrub 2 |
0.79 |
0.02 |
104% |
0.7 |
|
Strip 1 |
0.10 |
0.01 |
98% |
1.1 |
|
Strip 2 |
0.094 |
0.01 |
97% |
3.9 |
|
Strip 3 |
0.077 |
0.010 |
101% |
1.1 |
|
Tank 46F |
Dcs |
Std Dev |
Cs Recovery |
Std Dev |
|
Extraction |
12.4 |
0.57 |
90% |
0.21 |
|
Scrub 1 |
1.64 |
0.19 |
101% |
1.5 |
|
Scrub 2 |
0.71 |
0.01 |
103% |
1.1 |
|
Strip 1 |
0.10 |
0.023 |
99% |
1.3 |
|
Strip 2 |
0.082 |
0.008 |
93% |
3.2 |
|
Strip 3 |
0.073 |
102% |
4.8 |
|
|
Tank 34F |
Dcs |
Std Dev |
Cs Recovery |
Std Dev |
|
Extraction |
11.3 |
0.14 |
94% |
0.78 |
|
Scrub 1 |
1.86 |
1.19 |
101% |
1.8 |
|
Scrub 2 |
0.84 |
0.06 |
102% |
1.1 |
|
Strip 1 |
0.13 |
0.005 |
90% |
0.5 |
|
Strip 2 |
0.07 |
0.001 |
98% |
2.6 |
|
Strip 3 |
0.04 |
0.002 |
121% |
10.8 |
The scrubbing behavior was very repetitive for each of the batch tests for the two consecutive scrub steps. The distribution coefficient for the first scrub was between 1.64 and 1.86. Good agreement was obtained for each of the duplicate scrub tests for each of the tanks with the exception of Tank 34F where the difference for the averaged DCs value was high at 1.19. The value for the second scrub was always below 1 and ranged from 0.71 to 0.84. These distribution coefficients for scrubbing exceed the flowsheet basis of a value of 0.6. This behavior, however, does not match with the simulant data of Klatt where the second scrub results ranged between 1.16 and 1.91. The reduced scrub distribution coefficient will effectively lead to higher cesium concentrations in the extraction bank. In each of the scrubs the cesium accountability was within 5%.
The stripping distribution coefficients were acceptably low and well below the flowsheet requirement of 0.2. Previous testing had shown slightly higher values and it appears that the second scrub of the solvent neutralized the remaining caustic entrained in the solvent and allowed for efficient cesium stripping behavior. The first strip distribution coefficients were very low and near a value of 0.1. Again, with the exception of the third strip on the Tank 34F solvent samples the cesium radioactivity balance was very good.
ESS Behavior of 3H Evaporator Sample
On-going research programs in evaporator chemistry provided samples of supernate from the 3H Evaporator system (Tank 32H, feed tank and Tank 30H, drop tank). Only a limited quantity of material was obtained and this material had been passed through a 0.02 m m filter prior to the ESS testing. Unfortunately, there was insufficient sample to proceed past the second scrub test with the Tank 30H sample. Additionally, the Tank 32H composite was not counted prior to allocating the sample for the extraction tests. Therefore cesium accountability could not be completed.
The extraction distribution coefficients (Table 4) exhibited some variance and measured 9.7 and 11.1 for feed tank (32H) and the drop tank (30H), respectively. This result is a little surprising considering the amount of recycle between tanks that occurs when the 3H Evaporator operates. However, the extraction distribution coefficients are well above the flowsheet minimum of 8. The behavior during scrubbing also varied between the tanks. The scrubbing behavior for the Tank 32H sample was similar to the F-Area waste behavior previously discussed where the first scrub was near 1 and the second scrub value was below 1. In the data from the Tank 30H waste sample, both scrub measurements were above 1 and more in line with simulant measurements. The performance during stripping was acceptable for the sample from Tank 32H.
ESS Behavior of Permanganate-Treated Supernate and Dissolved Saltcake
Two additional ESS tests were performed on different waste types. The first waste type was a filtrate from a Cells Unit Filter demonstration run of a permanganate process. The permanganate process is an alternative strontium and actinide removal flowsheet under consideration as a replacement of the baseline monosodium titanate (MST) adsorption process. It had been previously shown that the MST process did not affect the cesium behavior during the CSSX process. Therefore, an ESS test was conducted on a filtrate sample from a composite tank sample that received the permanganate treatment. The second waste was a dissolved saltcake sample from Tank 37H that will be processed through the 2-cm centrifugal contactor assembly in the Shielded Cells.
Table 4. Batch Distribution Data for 3H Evaporator Tanks at 25°C
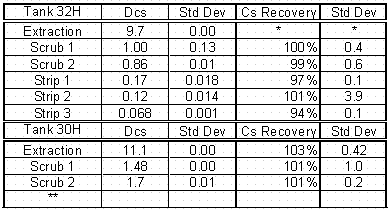
**Insufficient sample to continue testing
The behavior during the ESS testing for the permanganate-treated supernate comprised of samples from 37H, 44F, 26F, and 46F aligned well with the Tank 32H analysis results where the extraction distribution coefficient was near 9. The value of the first scrub distribution coefficient was above 1 with the second value slightly below 1. The stripping performance was again very successful. The data for the ESS testing with dissolved saltcake proved successful when compared to the flowsheet requirements of distribution coefficients of 8 for extraction, 0.6 for scrubbing and 0.2 for stripping. The extraction distribution coefficient was, however, lower than the values Walker measured in two other saltcake samples for Tank 38H and 46F. This may be an artifact of their solution chemistries. The chemistry of the Tank 37H saltcake showed higher levels of hydroxide than expected and higher than the samples tested by Walker
ESS Model Comparisons
Delmau and coworkers at Oak Ridge National Laboratory have been developing a multivariate thermodynamic model for predicting extraction behavior of cesium from solution comprised of a number of species present in SRS waste. In 2002, the model was extended to include behavior with the newly optimized solvent system and included additional species. The improved model was used to estimate the predicted extraction distribution coefficients for the wastes involved in this study. The data shown in Table 1 served as the basis for the chemical composition of the wastes. However, charge balance between the sum of cations and sum of anions was not satisfied. Therefore, slight changes in analyte concentrations were made prior to inputting the data into the thermodynamic model. Table 6 shows these chemical compositions.
Table 5. ESS Data from Testing with Permanganate Filtrate
and Dissolved Saltcake
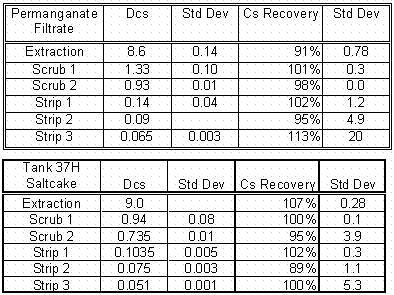
Table 6. Modified Chemical Compositions for Tank Waste Samples
The results of the modeling for the extraction distribution coefficients are shown in Table 7. For four of the seven actual waste matrices the model prediction and the experimental measurement agree within ~ 10%. The waste matrices are from Tanks 26F, 34F, 46F, and Tank 30H. For three waste matrices (Tank 32H, the permanganate-treated wasted composite, and the dissolved saltcake from Tank 37H) the variance is higher ~ 20 –30%. The authors reviewed the waste compositional changes performed as discussed above for their impact, i.e., a sensitivity analysis, and the resultant variance remained. There are no obvious compositional aspects that lead to the observed variance. The model improvements along with the experimental adaptations have increased the reliability of the model as a predictive tool for estimating the cesium behavior during the CSSX process.
Table 7. Comparison of Predicted and Measured Extraction
DCs
|
Tank Waste |
Predicted DCs |
Measured DCs |
|
Tank 26F |
14.27 |
12.7 |
|
Tank 34F |
11.51 |
11.3 |
|
Tank 46F |
13.70 |
12.4 |
|
Tank 30H |
12.26 |
11.1 |
|
Tank 32H |
12.17 |
9.7 |
|
MnO4 treat |
11.80 |
8.6 |
|
Tank 37H SC |
11.9 |
9.0 |
Conclusions
The CSSX process has been shown to effectively remove cesium for SRS high level wastes and provide the vitrification facility with a concentrated stream for coupled operations. Technical uncertainty exists due to the limited testing of the proposed flowsheet with actual waste. Therefore, an additional set of batch distribution tests of the CSSX process was performed with samples of supernate from the F- and H-Area tanks. Additionally, tests were performed with dissolved saltcake from Tank 37H.
Changes were executed in the experimental methodology used in the batch testing to improve experimental precision, reduce sample removal and lower cost. These changes produced an ESS protocol that reproducibly gave good cesium accountability. The measured performance for cesium using the newly optimized solvent system showed acceptable cesium behavior during the extraction, scrubbing and stripping steps in the CSSX processing. This indicates that the CSSX process will effectively decontaminate the high level waste and provide an adequately concentrated stream to DWPF.
The results also showed that the improved thermodynamic model predicts the cesium behavior during extraction. Moreover, the testing showed acceptable cesium behavior for two other actual tank waste matrices. These matrices included dissolved saltcake and permanganate-treated supernate.
References