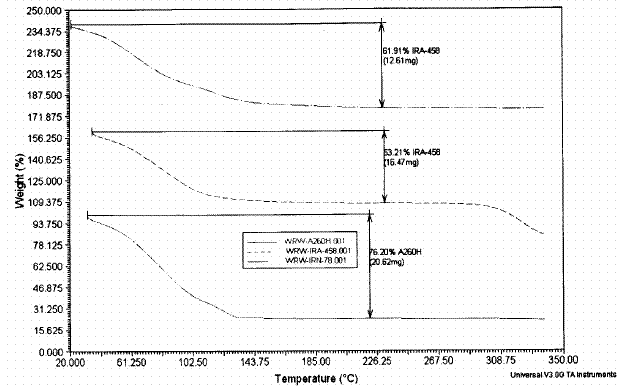
Figure 1. Thermal Analysis of Selected Resins
WSRC-TR-2002-00214
Silicon Removal from Waste Simulants via Ion Exchange
W. R. Wilmarth, J. T. Mills, and V. H. Dukes
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from:
U.S. Department of Commerce, National Technical Information Service, 5285
Port Royal Road, Springfield, VA 22161,
phone: (800) 553-6847,
fax: (703) 605-6900,
email: orders@ntis.fedworld.gov
online ordering: http://www.ntis.gov/help/index.asp
Available electronically at http://www.osti.gov/bridge
Available for a processing fee to U.S. Department
of Energy and its contractors, in paper, from:
U.S. Department of Energy, Office of Scientific and Technical Information, P.O.
Box 62, Oak Ridge, TN 37831-0062,
phone: (865 ) 576-8401,
fax: (865) 576-5728,
email: reports@adonis.osti.gov
Keywords: DWPF Recycle, Desilication, Ion Exchange
Summary
The Department of Energy’s Environmental Management office has funded a study to examine a number of silica removal technologies to assist the processing of DWPF recycle water. Ion exchange is used commercially to remove soluble silicate ions and colloidal silica from various process waters. Three candidate ion exchange resins were selected after a literature search showed a potential applicability for DWPF Recycle. The results of these scouting tests showed the resins to be chemically stable in the alkaline environment of DWPF recycle. However, the resins were not effective at removing silicon. Additionally, results of silica analyses showed the silicon solubility in these feed solutions for ion exchange were still high after further acidification with respect to the goal of silicon removal. This suggests very strongly that pH adjustment (from 14 to 9), as a silicon removal technology is not viable.
Introduction
The reaction between soluble silicate and aluminate ions in alkaline waste produces insoluble sodium aluminosilicate phases and has caused operation difficulty at the Savannah River Site (SRS).1,2 The aluminate concentrations in SRS wastes can range from a few hundredth molar to near 1 M and originated from the dissolution of aluminum-clad fuels and targets. Whereas, silicon concentrations have increased from ~ 25 mg/L to greater than 100 mg/L during the time that recycle water from the Defense Waste Processing Facility (DWPF) has entered the tank farm.3
The chemistry of silicates in alkaline environments has been studied for many decades and the chemistry is very complicated in the presence of competing ion such as aluminate.4 The compatibility of DWPF recycle was originally examined by Barnes5 but the study focused primarily on organic constituent interactions. After the discovery of aluminosilicate deposits, testing commenced to determine the kinetic relationship for the formation of sodium aluminosilicate.6,7 These tests showed that at elevated temperatures and highly supersaturated systems the rate of formation of aluminosilicate phases was very rapid.
As a results of these findings a multi-site team of researchers was assembled to investigate various aspects of the DWPF silica issue. Research programs at SRTC and Oak Ridge National Laboratory showed evaluated the effects of temperature and mixing on formation and deposition of aluminosilicate phases on metal surfaces.8 One aspect of the program was a literature search for various silicon control methods performed by Dr. E. J. Lahoda.9 Wilmarth incorporated the reported silica treatment methods from Lahoda’s study and others into a test plan.10 One method for silica removal identified in this testing plan was silica removal via ion exchange. This work was funded by the Department of Energy Tanks Focus Area.
Experimental Details
The target feed for silicon removal by ion exchange is the DWPF recycle that is stored in the H-Area Tank farm in Tanks 21H, 22H, and 24H. Therefore, reviewing the database of sample results for periodic sampling of these tanks provides the chemical compositions shown in Table 1. The simulant used in testing is a chemical composite of the H-Area tanks. The composite will be made from sodium salts of the various anions reaching a sodium ion concentration near 1M. The pH adjustment of the solution was made using 2 M nitric acid.
Table 1. Tank Waste Compositions (M)
|
21H |
22H |
24H |
Composite |
|
|
NO3- |
0.06 |
0.05 |
0.06 |
0.06 |
|
NO2- |
0.2 |
0.2 |
0.25 |
0.25 |
|
OH- |
0.5 |
0.5 |
0.5 |
0.5 |
|
Si |
0.002 |
0.0015 |
0.002 |
0.002 |
|
Al |
< 0.01 |
< 0.01 |
< 0.01 |
None Added |
|
CO32- |
0.04 |
0.05 |
0.01 |
0.075 |
Equilibrium distribution (Kd) tests were performed for 48 hours using 0.2 g of as-received resin and 20 mL of the salt solution. The solid-liquid mixture was shaken at 150 rpm using a New Brunswick orbital shaker at 25 ± 2 ° C. Solution phase silicon concentrations were measured by inductively coupled plasma – emission spectroscopy. Scanning electron microscopy was used to examine specimens of the resin before and after contact with the alkaline salt solution. The resin weights were corrected for water content. The water content was determined by thermal gravimetric analysis as shown in Figure 1. The percent water content of the selected resins was 61.9 % for the IRN-78 resin, 76.2 % for the A26 OH resin and 53.2 % for the IRA-478 resin.

Figure 1. Thermal Analysis of Selected Resins
Results and Discussion
Resin Selection
A literature search was performed by the SRTC technical library personnel. The search identified several applications of ion exchange technology for removal of colloids and silicates from industrial waters in the pH range of 8 to 10. There were no references identified for pH greater than 10. Therefore, the pH of the targeted solution had to be reduced from pH 14 and was accomplished as previously reported by the addition of dilute nitric acid.
Kunin and Hetherington11 reported as early as 1969 that marcoreticular resins such as Amberlite IRA-938 was effective at removing colloidal silica for water purification. Several references were found for reactor water clean-up. Within the United States, Elmiger, et al.,12 had shown the Rohm & Haas resin, IRN-78, was effective at removing silicon in slightly alkaline reactor water although the thrust of their work centered on boron removal. Additionally, Roma, et al.,13 showed that Ionac A-642 was effective at a pH of 8 in salt-bearing waters.
Several other researchers outside the U. S. had also shown effective ion exchange resins for removal of silicate ions and colloidal silica.14,15,16 showed several resins were effective for silicon removal. Resins that were evaluated included DIAION SA10A, IRA-420BL and IRA-478. After technical discussion with product support personnel, three resins were selected for evaluation. These resins were IRN-78, IRA-458, and a new product Amberlyst A26 OH.
Resin Stability
Specimens of the three selected resin were sent for analysis by scanning electron microscopy. Mircographs at various magnification of samples of each resin before and after exposure to the alkaline waste simulant are shown in Figure 2. For the A26 OH and IRA-458 resins there does not appear to be any physical changes in the resin beads after exposure to the alkaline salt solution nor does there appear to be any chemical attack on the resin bead. For the IRN-78 resin, the surface characteristic suggest chemical stability but the beads appear to have cracked. Swelling and shrinkage during the equilibrium distribution test probably cause the cracks. This phenomenon is well established in the literature and is a common problem with ion exchange resins.
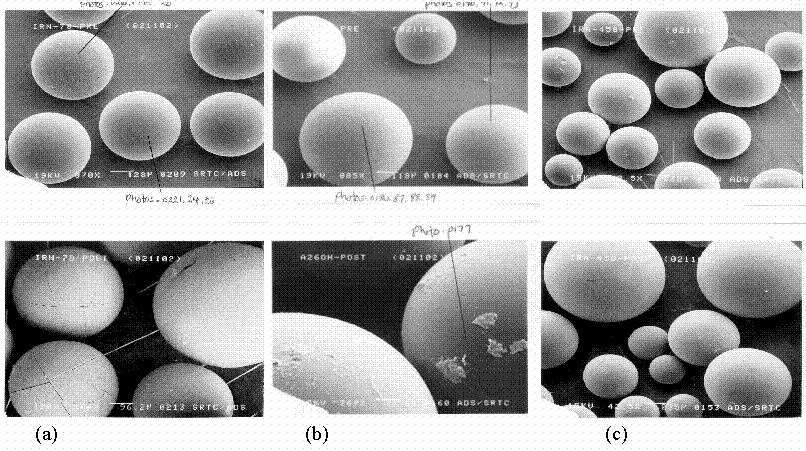
Figure 2. SEM Micrographs of the Selected Resins Before and After Exposure to the Alkaline Simulated Salt Solution.
The top photo in Set a is the IRN-78 pre-exposure sample (78x) and the bottom is
post-exposure (104x). Set b consists of pre- (85x) and post-exposure (269x) samples of A26 OH.
Set c consists of the pre- (48.5x) and post-exposure (42.5 x) samples for IRA-458.
Silicon Removal Performance
Batch equilibrium distribution tests were performed for 24 and 48 hours under the conditions previously described. Liquid phase samples were pulled at the completion of the test and filtered through 0.45 m m filters prior to submission for ICP-ES analysis. Table 2 contains the test-specific information.
Table 2. Silicon Kd Test Conditions
|
Resin |
Test No. |
Wt (g) |
Corrected Wt (g) |
||
|
IRN-78 |
1 |
0.202 |
0.077 |
||
|
IRN-78 |
2 |
0.212 |
0.081 |
||
|
A26OH |
1 |
0.207 |
0.049 |
||
|
A26OH |
2 |
0.214 |
0.051 |
||
|
IRA-458 |
1 |
0.198 |
0.093 |
||
|
IRA-458 |
2 |
0.203 |
0.095 |
||
|
Volume |
Temperature |
Shaking Speed 150 rpm |
|||
|
20 mL volumetric |
25 ± 2 ° C |
||||
The Tank 20 simulant contained averaged 51.3 ± 0.5 mg/L of silicon as prepared. The solution was neutralized with 2 M nitric acid to a pH of 9 and re-measured for silicon concentration. The neutralized, Tank 20 simulant as feed to the Kd tests averaged 49.6 ± 0.3 mg/L. This information serves two aspects of testing. First, the measurements provide the starting conditions for the ion exchange tests. Secondly, one of the possible treatment technologies for silicon removal was pH adjustment. It is clear from these measurements that adjusting pH from 14 to the lower tank limit (pH 8-9) will not effectively reduce the silicon concentration low enough to allow processing in all site evaporators.
Figure 3 shows the results of the 24 and 48 hour ion exchange tests. In each set of tests there is no evidence of silicon removal. The calculated Kd’s ranged from 0 to 3 mL/g. As previously stated the resin was stable in the alkaline solution. There was only limited data in the literature and predominantly in low ionic strength solutions. The high ionic strength may reduce the selectivity for silicate over the other ions present e.g., nitrate, nitrite.
Conclusions
Research efforts are on-going to examine a number of silica removal technologies to support processing options for DWPF recycle. Ion exchange is one of those technologies and is used commercially to remove soluble silicate ions and colloidal silica from various process waters. Three candidate ion exchange resins were selected after a literature search showed a potential applicability for DWPF Recycle. The results of these scouting tests showed the resins to be chemically stable in the alkaline environment of DWPF recycle. However, the resins were not effective at removing silicon. Additionally, during the course of these tests a pH adjustment step was performed to lower the pH to between 8 and 10. Results of silica analyses showed the silicon solubility in these feed solutions for ion exchange were still high with respect to the goal of silicon removal. This suggests very strongly that pH adjustment from 14 to 9, as a silicon removal technology, is not viable.
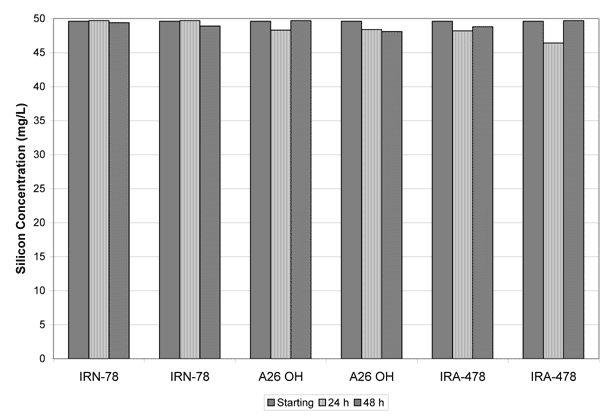
Figure 3. Results of Ion Exchange Tests for Silicon Removal
Acknowledgements
The authors wish to acknowledge several people for their help and assistance in this task. Dr. F. F. Fondeur provided the thermal analysis and Susan Isaacs-Bright performed the literature search. The Analytical Development Section performed the silicon analysis on submitted samples along with the characterization of resin samples.
References