WSRC-TR-2002-00026
Stability Tests with Actual Savannah River Site Radioactive Waste
D. D. Walker
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/help/index.asp
Available electronically at http://www.osti.gov/bridge
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Keywords: Waste Processing, Aluminum, Precipitation
Abstract
Researchers investigated the stability of Savannah River Site (SRS) radioactive waste solutions in two laboratory experiments. The first experiment tested four waste solutions for supersaturation of aluminum by monitoring the aluminum concentration after seeding with gibbsite. The second experiment tested two waste samples for precipitation of aluminosilicates by heating the solutions to accelerate solids formation. The results of the experiments with actual waste solutions support the following conclusions.
- The soluble aluminum concentration in Tank 30H waste did not change
and in Tank 34F and Tank 37H/44F composite waste the soluble
aluminum concentration increased when seeded with gibbsite.
- Evaluation of the Tank 38H and 43H compositions by a Geochemist
Workbench solubility model suggest that solids should not have formed during
the test.
Previous studies showed simulated waste solutions exhibit both instabilities.1 The absence of aluminosilicate formation in actual waste samples should not be taken as an indication that this cannot occur during waste processing. Aluminosilicate formation in SRS evaporators is well documented.2-4
The current flowsheet specifications for waste dilution are inadequate to prevent gibbsite formation in most tank wastes and greatly underestimate the amount of dilution required. Since aluminosilicates and gibbsite form slowly and therefore may not accumulate rapidly in the contactors, it is recommended that nitric acid cleaning of contactors be considered as a less expensive alternative to waste dilution.
Introduction
The Department of Energy identified caustic-side solvent extraction (CSSX) as the preferred option for removing cesium from Savannah River Site high activity waste prior to disposal.5 Suspended solids in the feed stream represent an important operational concern for a solvent extraction process using centrifugal contactors. Recent work by ORNL researchers showed that suspended solids collect in the contactor stages.6 Continued accumulation will adversely affect hydraulic performance. At present, the conceptual design for the process includes filtration of the waste solution prior to CSSX processing.7 In addition, the baseline flowsheet requires dilution of the waste stream with 1.6 M NaOH in an effort to prevent precipitation following the filtration step. Previously, tests demonstrated simulated waste solutions commonly used in the Salt Processing Project research and development programs8 are unstable with respect to slow-forming solid phases.1 Although such solutions may appear stable for many months (i.e., aluminum concentrations do not significantly change), seeding with gibbsite accelerated precipitation of aluminum hydroxide. Gibbsite is the thermodynamically stable form of aluminum hydroxide, Al(OH)3, below 155 ° C. Bayerite is the stable form above 155 ° C. The aluminum concentration in average and high nitrate simulated waste solutions seeded with gibbsite slowly decrease over several months at ambient temperatures. Additionally, aluminosilicates (such as Zeolite-A, hydroxysodalite, and cancrinite) form when waste solutions contain sufficient amounts of silica. The present study investigated actual waste solutions for the same instabilities observed in simulants.
This work partially fulfills item 7.2.3.7 in the Tank Focus Area research and development program for CSSX described by H. Harmon, R. Leugemors, S. Schlahta, S. Fink, M. Thompson, and D. Walker in "Savannah River Site Salt Processing Project: FY 2002 Research and Development Program Plan," PNNL-13707, Rev. 1, December, 2001. The work complies with the following plan: D. D. Walker, W. R. Wilmarth, F. F. Fondeur, and T. Hang, "Technical Task and Quality Assurance Plan for Non-Elutable Ion Exchange Process Waste Stability and IONSIVÒ IE-911 Performance Tests," WSRC-RP-99-01079, Rev.0, December 20, 1999.
Results
Waste Sample Origin and Composition
Researchers tested six SRS radioactive waste samples. The four samples used in the aluminum solubility tests (Tanks 30H, 32H, 34F, and 37H/44F composite) came from previous SRTC test programs. Unused portions of Tank 30H, 32H, and 34F waste solutions prepared in September, 2000, remained following ion exchange batch distribution tests with IONSIVÒ IE-911.9 Researchers had diluted the original Tank 30H, 32H, and 34F waste samples using water. Unused portions of the Tank 37H/44F composite sample prepared in February, 2001, remained from a solvent extraction centrifugal contactor test.10 Researchers had diluted the Tank 37H/44F composite waste sample using 2.0 M NaOH solution. Table I lists the previously reported compositions of the four samples. As shown in Table I, the samples contained approximately 5.6 M Na+ (or less) and required no further dilutions or adjustments prior to use.
Two new samples (Tank 38H and 43H), obtained in dip bottles in July, 2001, served for the heating tests. At the time of sampling, Tanks 38H and 43H served as the 2H Evaporator drop tank and feed tanks, respectively. Researchers selected these tanks because aluminosilicate plugging problems in the 2H evaporator suggested the waste in the tanks should exhibit instability toward solids formation.11 The low salt concentration in the drop tank (2.7 M Na+) compared to the feed tank (5.7 M Na+) resulted from flush water used in the evaporator and gravity drain line and is consistent with other samples taken during 1999 and 2000.11 Researchers determined the composition of the new samples by routine analyses provided by the SRTC Analytical Development Section. Table I lists the waste compositions. Because of the low sodium ion concentration, we tested the samples without dilution. Appendix A provides additional details of their origin and analysis.
Radioactive Waste Stability Tests
The stability tests examined six SRS high-level radioactive waste samples for precipitation of solids induced by seeding with gibbsite or heating at 90 ° C. Researchers used test protocols similar to those reported previously with simulated SRS wastes.1 Appendix A provides additional details.
Aluminum Solubility Test
In this study, we tested four high-level radioactive waste samples for supersaturation with respect to aluminum. The tests initiated by adding gibbsite crystals to the waste solution at ambient temperature (23± 5 ° C). Figure 1 shows the changes in the soluble aluminum concentration during the 115 days following addition of gibbsite seeds. See Appendix A, Table A-II, for a tabular list of results. We observed all three possible changes in the aluminum concentration: soluble aluminum decreased in one sample, remained unchanged in one sample, and increased in two samples. Although the majority of the change occurred in the first 20 to 40 days, minor differences in concentration between 64 and 115 days may indicate the solutions did not reach equilibrium by the end of the test.
Table I. Composition of Radioactive Waste Solutions

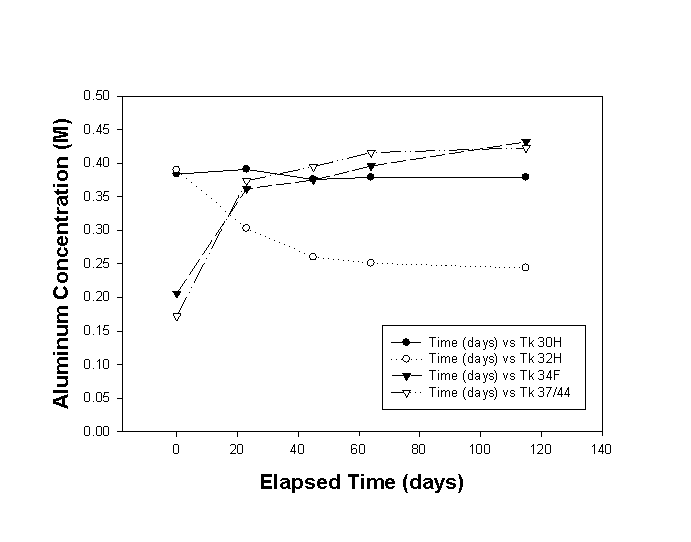
Figure 1. Results of Aluminum Seeding Test
The aluminum concentration in the Tank 32H solution decreased by 37% following seeding. The Tank 30H solution did not change significantly (<3%). Two waste samples increased in aluminum concentration: Tank 34F by 110% and Tank 37H/44F by 144%.
Note that the Tank 32H solution stood for six months or longer without significant change in the soluble aluminum concentration measured prior to seeding with gibbsite.
Heat Treatment Test
In this study, we tested two samples of radioactive waste for precipitation of aluminosilicate compounds. Researchers heated ~250-mL samples of radioactive waste at 90 ° C to initiate growth of solids. Heating occurred at atmospheric pressure for 24 hours in a stainless steel vessel fitted with a glass condenser. See Figure A-1 in Appendix A for details. The condenser prevented evaporation and concentration of the samples. After cooling the treated samples to ambient temperature overnight, researchers examined the solutions for cloudiness, inspected the walls of the stainless steel vessel for deposited solids, and analyzed the nitric acid solutions used to clean the stainless steel vessels. No solids appeared on the sides of the vessel or in the solutions. Table II lists the results from the analyses of the wash solutions. The table also lists calculated concentrations in the wash solution based on dilution of a small amount of waste or based on dilution of the same small amount of waste with dissolution of 10 mg of hydroxysodalite. The observed concentrations in the wash solutions correspond closely to simple dilution of waste and suggest that less than 10 mg of hydroxysodalite dissolved. This result also indicates no Zeolite-A or cancrinite formed because they have similar Na:Al:Si ratios to hydroxysodalite.
Table II. Na, Al, and Si Concentrations in Wash Solutions
|
Concentration (mg/L) |
|||
|
Na |
Al |
Si |
|
|
Tk 38 wash (found) |
1175 |
26.4 |
1.5 |
|
Tk 38 calculated for 4.7 mL waste |
1167 |
26.9 |
2.9 |
|
Tk 38 calculated for 4.7 mL waste and 10 mg hydroxysodalite* |
1175 |
33.6 |
9.9 |
|
Tk 43 wash (found) |
1274 |
32.3 |
2.2 |
|
Tk 43 calculated for 2.4 mL waste |
1267 |
31.1 |
1.6 |
|
Tk 43 calculated for 2.4 mL waste and 10 mg hydroxysodalite* |
1275 |
37.8 |
8.6 |
|
____________________________ |
|||
Discussion
The results of the waste stability tests and evidence from tank farm evaporator operations indicate that radioactive waste exhibits instability toward precipitation of solids. The types of instability observed in radioactive waste are also observed in simulated wastes and include supersaturation with respect to aluminum and precipitation of aluminosilicates.
Aluminum Precipitation
The Tank 32H waste sample examined in this study exhibited supersaturation with respect to aluminum. Seeding with gibbsite, the thermodynamically stable form of Al(OH)3 below 155 ° C, resulted in a 37% decrease in the soluble aluminum concentration over a period of 115 days. Although we did not analyze the solids at the end of the test, aluminum likely precipitated as gibbsite. Prior to seeding, this solution remained at ambient temperatures (23± 5 ° C) for 280 days without reaching equilibrium or changing significantly in aluminum concentration.
Previous testing demonstrated that two of the three simulated SRS waste recipes commonly used in Salt Processing Project research and development programs are unstable toward precipitation of aluminum.1 Although the simulants appear stable for many months (i.e., aluminum concentrations do not significantly change), seeding with gibbsite resulted in decreases in the soluble aluminum concentration.
Previous modeling results from ORNL using the SOLGASMIX computer code indicated supernate solutions in several SRS waste tanks and twelve of the thirteen annual average compositions7 for the salt waste feed stream should precipitate gibbsite.12 The ORNL model also correctly predicted aluminum supersaturation in the Tank 32H sample examined in this study (see Appendix B).
Aluminosilicate Precipitation
Researchers did not detect precipitation of aluminosilicate compounds in two waste samples heated to 90°C for 24 hours. The negative results were unexpected since the two samples came from an evaporator system that recently plugged from aluminosilicate formation. Previous work with simulants demonstrated that the heat treatment method used in these tests causes formation of detectable amounts of aluminosilicate compounds.1 The negative results with the Tank 38H and 43H wastes could be due to insufficient sensitivity of the detection methods. However, solubility modeling results indicate that the Tank 38H and 43H waste samples in this study have compositions that should not produce solids (Appendix B). In view of the evaporator plugging incidents, the negative results from the current tests should not be interpreted to mean that aluminosilicates cannot form from SRS waste.
For samples taken during the time that plugging occurred in the 2H Evaporator system, the Geochemist Workbench model correctly predicted precipitation of aluminosilicates.11 Analysis of samples taken after the plugging and prior to the date of the current samples (i.e., in June 2001), indicated the waste composition changed sufficiently to make solids formation less likely.11, 13 Modeling predicted that aluminosilicates should not form in the feed tank waste (i.e., Tank 43H) but could form in the drop tank (i.e., Tank 38H). Additional modeling calculations using the specific compositions of the samples used in this study (from June 2001) suggest aluminosilicates would not form by heating for 24 hours at 90°C (Appendix B).
Dilution of SRS Waste with NaOH
The following discussion examines the usefulness of sodium hydroxide dilution of SRS waste to avoid post-filtration precipitation of solids in a solvent extraction process. Tank farm experience, waste and simulant testing, and modeling calculations suggest that aluminum precipitation and aluminosilicate formation are the most likely forms of post-filtration solids formation. Avoiding accumulation of solids in the contactors is necessary to ensure adequate performance. However, other potential solutions to the problem may avoid the extra expense and process complexity added by sodium hydroxide dilution.
The current salt processing flowsheet for solvent extraction includes dilution of radioactive waste feed (6.44 M Na+) with sodium hydroxide solution (1.66 M) to achieve the desired salt concentration (5.6 M Na+) for processing.7 Including NaOH in the dilution water decreases the tendency for post-filter precipitation of gibbsite. The prevention of gibbsite formation derives from both the decrease in the aluminum concentration by dilution and the increase in pH from adding sodium hydroxide. Dilution with sodium hydroxide solution increases the volume of waste to Saltstone by approximately 5% compared to dilution with water. This corresponds to an annual Saltstone cost of approximately $1,200,000 (based on 6 million gallons of waste processed per year at $4.01 per gallon14). These figures are based on an average waste composition over the 13 years required to process the waste.
Using the SOLGASMIX model, C. F. Weber12 calculated the amount of NaOH dilution required to prevent gibbsite formation for the predicted waste compositions for each of the 13 years of salt processing. The model predicts gibbsite formation in 12 of the 13 years. In only one of the 12 years would the flowsheet dilution actually prevent the formation of gibbsite. In the other 11 years, significantly more NaOH and dilution volume are required to avoid gibbsite formation. Weber's calculations indicate 2X to 22X more kilograms of NaOH are required and 9 to 30% more saltstone would form than indicated by the flowsheet. Thus, the flowsheet dilution plan greatly underestimates the amount of dilution required to prevent gibbsite formation. The actual costs to ensure gibbsite will not form may be as high as $2 to $7 million per year.
An alternative exists for preventing solids accumulation in the contactors. Slow accumulation of gibbsite and aluminosilicates could be controlled by periodic acid washing of the contactors. Nitric acid, which is already used in the process, dissolves gibbsite and aluminosilicates. For example, 2 M nitric acid removed deposits of aluminosilicates in the 2H Evaporator.15 Nitric acid cleaning may also remove accumulated sludge and allow strontium and actinide removal following (rather than ahead of) solvent extraction. The frequency of cleaning is one of the significant factors in a decision to use this method. Current knowledge suggests that precipitation of solids remains slow at process temperatures with little solids formation likely. Thus, the frequency of cleaning may be low. Additional evaluation of this process option is recommended.
Summary and Recommendations
Researchers investigated the stability of SRS radioactive waste solutions in two laboratory experiments. The first experiment tested four waste solutions for supersaturation of aluminum by monitoring the aluminum concentration after seeding with gibbsite. The second experiment tested two waste samples for precipitation of aluminosilicates by heating the solutions to accelerate solids formation. The results of the experiments with actual waste solutions produced the following results.
The absence of aluminosilicate formation in actual waste samples should not be taken as an indication that this cannot occur during waste processing since aluminosilicate formation in SRS evaporators is well documented.2-4
The current flowsheet specifications for waste dilution are inadequate to prevent gibbsite formation in most tank wastes and greatly underestimate the amount of dilution required. Since aluminosilicates and gibbsite form slowly and, therefor, may not accumulate rapidly in the contactors, it is recommended that the program evaluate alternatives to waste dilution.
The following areas of investigation are recommended to support an evaluation of alternatives to waste dilution.
Acknowledgments
The author thankfully acknowledges the contribution of Mary H. Beasley, Alda Coleman, and Betty H. Croy who performed the experiments described in this report. In addition, I thank James E. Laurinat (SRTC), John M. Pareizs (SRTC), and Charles F. Weber (ORNL) for the solubility calculations for aluminum and aluminosilicates. Carol M. Jantzen (SRTC) provided many helpful discussions on the construction and interpretation of activity diagrams.
References
Appendix A
Experimental
Characterization of Waste Solutions
The SRS tank farm personnel provided samples of liquid radioactive waste. Table A-I lists the origins of the samples used in this study. In general, multiple samples taken in 100-mL stainless steel dip bottles from the indicated tank or tanks were combined in a single polyethylene sample bottle. The Tank 30H, Tank 32H, and Tank 34F samples were diluted with water to achieve a final sodium ion concentration representative of the proposed process target (5.6 M Na+). The Tank 37H/44F composite sample derived from the combination of two 38-L samples and was diluted with 2 M NaOH to reach 5.6 M Na+. The Tank 38H and Tank 43H solutions were used without dilution. Researchers measured the densities of the solutions at ambient temperature (23± 5°C) using 50- or 100-mL volumetric flasks and an analytical balance (± 0.001 g). They also diluted 1-mL aliquots gravimetrically in water or 0.1 M nitric acid to reduce the radiation dose rates prior to removing portions from the shielded facility for analysis. Personnel in the SRTC Analytical Development Section analyzed the solutions using routine methods.
Aluminum Seeding Test
Researchers placed waste samples (75 mL) into polyethylene bottles containing stir bars and 2.0 g of gibbsite powder (Baker, Lot#423359). The slurries stirred at ambient temperatures (23± 5°C) for 115 days. Periodically, 5 mL samples were pulled from the bottles, filtered through syringe filters (Acrodisc, nylon, 0.45 micron nominal pore size), diluted 100-fold in 0.1 M nitric acid, and a portion analyzed for aluminum concentration by inductively coupled plasma emission spectroscopy. Table A-II lists the results of the analyses corrected for the dilution in acid.
Table A-I Origin of Radioactive Waste Samples
Table A-II. Results of Aluminum Seeding Test*
|
Elapsed Time |
Aluminum Concentration (M) |
|||
|
(days) |
Tk 30H |
Tk 32H |
Tk 34F |
Tk 37H/44F |
|
0 |
0.384 |
0.390 |
0.206 |
0.172 |
|
23 |
0.391 |
0.303 |
0.362 |
0.374 |
|
45 |
0.376 |
0.260 |
0.375 |
0.395 |
|
64 |
0.379 |
0.251 |
0.396 |
0.416 |
|
115 |
0.379 |
0.244 |
0.432 |
0.420 |
*See Figure 1 for a graphical representation of these results.
Heating Test
Researchers filtered the combined dip bottle samples listed in Table A-I, placed the majority of the filtrate (~225 mL) into a stainless steel container fitted with a glass reflux condenser (Figure A-1), and heated the solutions at 90 ° C for 24 hours. After cooling to ambient temperature overnight, researchers visually inspected the stainless steel vessel
and salt solution but detected no solids. Researchers then removed the salt solutions and cleaned the vessels with 250 mL of 1.5 M nitric acid by heating at 90 ° C overnight. Table A-III lists analytical results for the cleaning solutions and a blank sample. The blank sample consisted of 250 mL of 1.5 M nitric acid heated in the stainless steel vessel prior to the first waste sample.
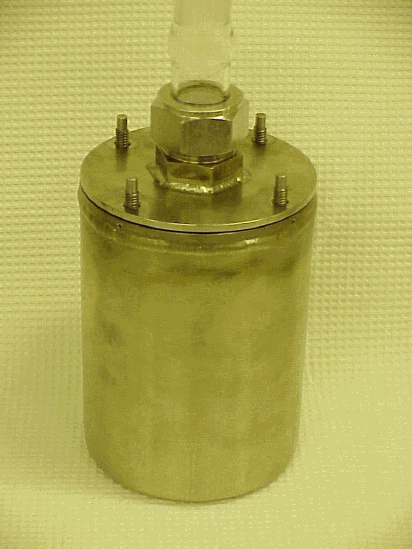

Figure A-1. Reflux Apparatus for Heat Treating Solutions
Table A-III. Analytical Results for the Cleaning Solutions from
Heating Tests
|
Sample |
Concentration (mg/L) |
|||
|
Na |
Al |
Si |
Fe |
|
|
Blank* |
1.44 |
1.82 |
1.73 |
35 |
|
Tk 38H |
1175 |
26.4 |
1.54 |
0.3 |
|
Tk 43H |
1274 |
32.3 |
2.21 |
10 |
Appendix B
Solubility Calculations
Table B-I lists the composition of the six solutions used in the calculations. These differ slightly from the compositions listed in Table I. The sodium ion concentrations in Table I were adjusted to achieve charge balance with the anion results. Charles F. Weber of ORNL used the SOLGASMIX code12 to calculate the aluminum solubility in the first four waste solutions. Table B-II lists the predictions. The model correctly predicted two of the solutions (Tk 34F and Tk 37H/44F composite) were undersaturated and one solution was supersaturated (Tk 32H). The fourth solution (Tank 30H) appeared at saturation in the seeding test, but was predicted supersaturated by 34%.
Table B-I. Waste Solution Compositions Used in Solubility
Calculations

Table B-II. SOLGASMIX Aluminum Solubility Predictions
|
Soluble Aluminum (M) |
||||
|
Tank |
30H |
32H |
34F |
37H/44F |
|
SOLGASMIX prediction |
0.29 |
0.19 |
>0.20 |
>0.17 |
|
Observed* |
0.38 |
0.24 |
0.43 |
0.42 |
*Concentration after 115 days (see Figure 1). The solutions likely were not at equilibrium.
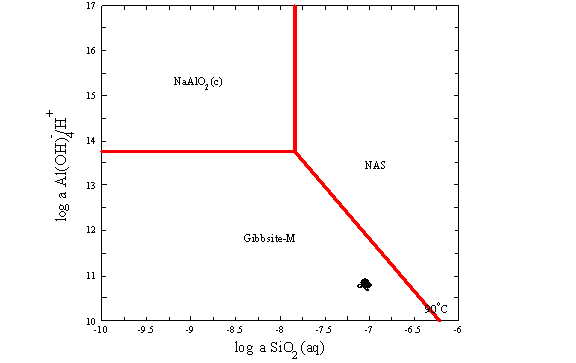
James E. Laurinat and John M. Pareizs of SRTC used Geochemist Workbench to predict precipitation of aluminosilicates in the Tank 38H and 43H two waste solutions.11,16 Figure B-1 shows the results. The compositions of the two waste samples do not fall within the stability field of sodium aluminosilicate hydrogel (NAS) at 90 ° C. However, they do fall within the stability field for mixed zeolite solid (not shown). NAS is therefore not predicted to form in the experiment because it is the initial solid phase formed from waste solutions in the following sequence of transformations.
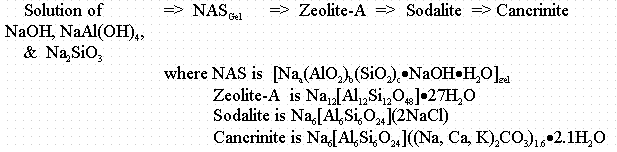
In waste solutions containing low concentrations of chloride and high concentrations of hydroxide and nitrate ions, hydroxysodalite or nitrated sodalite form. These compounds have the same aluminosilicate cage structure as natural sodalite, but contain hydroxide or nitrate ions replacing the chloride. Cancrinite undergoes similar anionic substitutions.
Tank 38H at 90 ° C
Tank 43H at 90 ° C
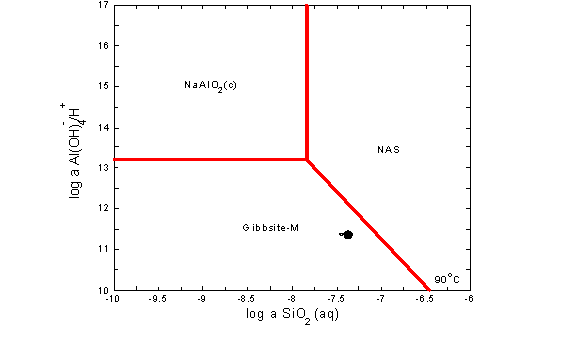
Figure B-1. Activity Diagrams for Tanks 38H and 43H Waste Samples