WSRC-TR-2001-00582
Tank 38H Desilication Study
W. R. Wilmarth, J. T. Mills,
and V. H. Dukes
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/index.html
Available electronically at http://www.osti.gov/bridge
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Summary
In support of potential option for treating high level waste supernates that have elevated levels of silicon, a series of tests was performed to measure the rate of desilication of High Level Waste simulants as a function of time at an elevated temperature (50°C).These studies indicate that the addition of aluminate ion to typical wastes like Tank 38H supernate can reduce the soluble silicon concentration. Previous work in highly supersaturated simulants indicated reaction times would be on the order of days to weeks. In this study, we examined simulants that were only mildly supersaturated with respect to aluminosilicate formation. Because the silicon concentration was limiting, the desilication data fit to pseudo first order reaction kinetic expressions. It was determined that the reaction rate constant was affected by the degree of supersaturation. This indicated that the reaction times to complete desilication of a high level waste supernate may be much longer than expected and may approach month-long time frames at 50°C. Additionally, the data were examined in terms of crystal growth kinetics used by Addai-Mensah and other researchers in the aluminum industry. The order of crystal growth observed in these tests with a Tank 38H simulant was 1 and was similar to that measured for industrial simulants.
Keywords: Aluminosilicate, Reaction Kinetics, Aluminum Addition
Introduction
Over the past several years, a number of studies1,2,3 examined the formation of aluminosilicates in Savannah River Site High Level Waste supernate as a result of the scaling observed in the 242-16H (2H) Evaporator. Additionally, thermodynamic modeling efforts4,5,6 enabled personnel to identify tank conditions that favor the formation of aluminosilicates. These studies concluded, that during the operation of the 2H Evaporator with aluminum concentrations from H-canyon waste transfers and silicon concentrations from DWPF recycle waters, conditions were optimal for the formation of aluminosilicate.
With the curtailed operation of the 2H Evaporator system, the feed and drop tanks, Tanks 43H and 38H, respectively, were initially supersaturated with aluminum and silicon. Analysis7 of samples from the feed tank, Tank 43H, showed the reaction to form aluminosilicate continued, thereby depleting the levels of supersaturation. The drop tank, however, had not reached equilibrium with respect to aluminosilicate formation.
Therefore, a series of tests were proposed8 to examine an aluminum strike to further react the soluble silicate species. The resultant silicon-depleted supernate would be suitable for processing in other site evaporators, e.g., 2F or 3H Evaporators. Sampling of the liquid phase for the aluminum and silicon concentration with time is analyzed to determine the reaction kinetic relationship.
Experimental
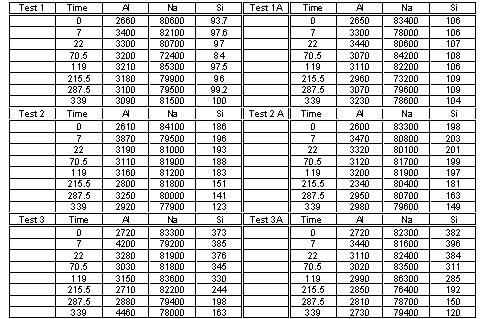
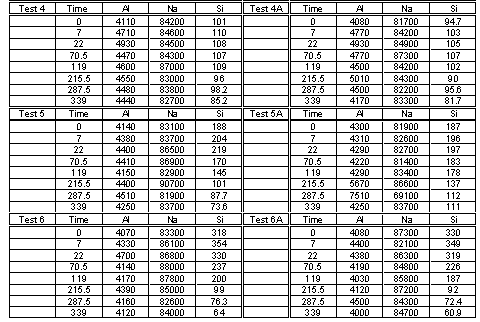
Aluminosilicate formation tests were conducted in Tank 38H waste simulants.9 These tests were performed on a nominal 100-mL scale at 50°C in Teflon bottles. Periodically, liquid samples were removed by syringe and filtered through 0.02 m m pore sized filters. Thereafter, the liquid samples were submitted for elemental analysis using Inductively Coupled Plasma – Emission Spectroscopy. In order to measure the desilication kinetics accurately, silicon, in the form of sodium metasilicate, was added to the simulant at three levels and the reactant aluminum was added at two levels as shown in Table 1.
Table 1. Simulant Composition and Test Matrix
|
Analyte |
Concentration |
|||||
|
Na+ |
4 M |
|||||
|
OH- |
2 M |
|||||
|
NO3- |
1 M |
|||||
|
NO2- |
1 M |
|||||
|
Al |
2000 mg/L |
|||||
|
Test No. |
||||||
|
1 |
2 |
3 |
4 |
5 |
6 |
|
|
Si (mg/L) |
100 |
200 |
400 |
100 |
200 |
400 |
|
Al (mg/L) |
500 |
500 |
500 |
2000 |
2000 |
2000 |
Laboratory notebooks WSRC-NB-2001-00002 and -00064 contain data obtained during these tests and the procedures used.10 Personnel used routine analytical protocol for the samples in this report.11 Each test was performed in duplicate and the analytical results are shown in Table 2. Further data analysis was performed on the average of either the silicon or aluminum concentrations for each test.
Table 2. Measured Analyte Concentrations (mg/L) versus Time (h)


Results and Discussion
The hydrothermal formation of sodium aluminosilicate under the highly caustic condition of the Tank 38H simulant is thermodynamically favored.12 At elevated temperature (> 80°C), our experience is the chemical reaction shown in Equation 1 is rapid, however, the work of Wilmarth1 and Mattus3 using high level waste simulants studied the formation of sodium aluminosilicate from highly supersaturated systems. Tank 38H, on the other hand, is slightly supersaturated if one wished to add aluminate ion to deplete the silicon supersaturation. The tests reported herein examined small aluminate ion additions to two Tank 38H simulant solutions with relatively low silicon concentrations
6 AlO4- + 6 SiO32- + 2 NO3- + 8 Na+ ® Na8Al6Si6O24(NO3)2 Eq. 1
Shown in Figure 1 are the plots of desilication in each of the six tests. Reaction, i.e., loss of silicon from solution, was observed in four of the six tests. The silicon concentrations in Test 1 and 4 did not significantly decrease during the nearly 350 h test. These tests (1 and 4) had the lowest level of starting silicon concentration (100 mg/L). The pseudo solubility product constant, Ksp, for these tests were ~ 3.5 x 10-4 M2 which is low and most probably near a supersaturation index, s , of 0 where s = (C0 –Ceq)/Ceq.

Figure 1. Averaged Silicon Concentrations in the Desilication Tests
In the other tests (2, 3, 5, and 6), silicon concentrations in the supernate phase is observed to decrease over the 340 h test duration. In tests 3 and 6, where the starting concentrations is the highest, the concentration of silicon drops from above 350 mg/L to below 100 for test 6 and below 200 for test 3. This silicon reaction behavior is, however, different from the observations of Mattus. In those tests at a slightly higher temperature (60°C), silicon was consumed within 30 hours.
Previously, Mattus3 examined the reaction kinetics of the aluminosilicate formation in SRS simulants and found the data for the silicon and aluminum concentrations fit a standard integrated form for a second order reaction equation. With equal molar starting concentrations this second order equation has the form shown in Equation 2. In that examination of the reaction kinetics, when the aluminum concentration approached a 10-fold excess to the silicon concentration, Mattus noted that the kinetic relationship could be fitted using pseudo first order equations and is how Wilmarth1 treated initial experimental data.
dx/dt = k ( a – x )2 Eq. 2
where x = concentration, t = time
and a = initial concentration
Therefore, the silicon data for the Tests 2, 3, 5 and 6 were regressed using pseudo first order kinetic equation (Equation 3). In this analysis, one plots the natural logarithm of the concentration of silicon versus time. The slope of the fitted line is equal to the rate constant. Shown in Figure 2 are the results of this regression for Test 6. The regression fits the data very well. The measured rate constant is 0.0053 h-1 at 50°C. The magnitude of this rate constant is about a factor of 2 below previously measured1 pseudo first order rate constants (0.017 h-1) at 40°C.
ln c = - k t + C Eq. 3
where c = concentration, k = rate constant
t = time and C = integration constant
Regression of the silicon data from the other tests gave rate constants of 0.001, 0.0029, and 0.0024 h-1. Because all tests were performed at the same temperature, these values for the rate constants do not appear to agree within this data set and with other data sets form other tests performed in similar matrices. However, it must be recognized that applying these types of kinetic relationships are normally performed when the reacting species and product species are the same phase. In these tests for aluminosilicate formation, the soluble reacting species condense and precipitate as a solid aluminosilicate phase. Perhaps the driving force for the reaction changes with another parameter.
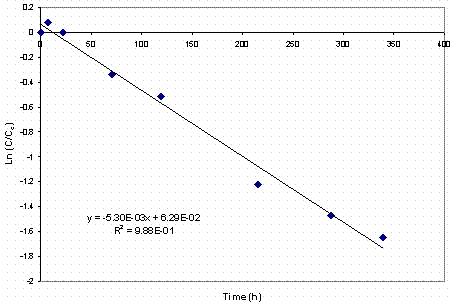
Figure 2. Kinetic Plot of Silicon Data from Test 6
Shown in Figure 3 is a plot of the measured pseudo first order rate constants for Tests 2, 3, 5, and 6 plotted against the starting supersaturation index, s . As previous mentioned, the supersaturation index is calculated by s = (Co – Ceq)/Ceq. In our analysis of the data, Ceq was approximated by Cfinsl. The plot in Figure 3 shows a linear relationship between the degree of supersaturation or supersaturation index and the rate constant. The fit of the linear expression to the data is good but some variance from the fit is observed. However, it makes common sense that in the reactions to form aluminosilicate the kinetic driver to reach equilibrium is the need to deplete the supersaturation. As the driver is lowered, i.e., degree of supersaturation is lower, than the reaction kinetics are slower.
The implication of this relationship between reaction rate constant and degree of supersaturation is that the reaction time duration may be longer than expected if based on previous work. Initially, the reaction half life was expected to be 1 week; thus, in three weeks nearly 90% of the reaction would be complete. However, if the reaction starts with s = 2, it will take 4.5 weeks to reach 90% reaction or ~7 weeks for s = 1.
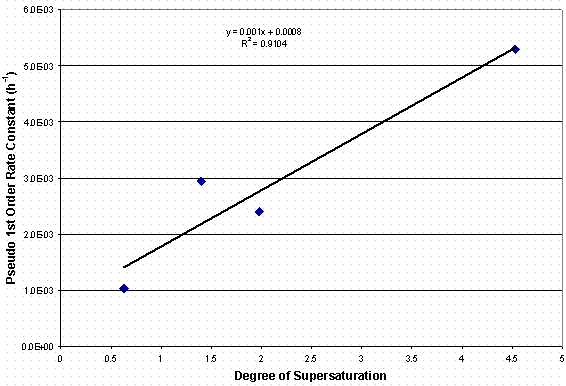
Figure 3. Plot of Rate Constant vs. Degree of Supersaturation
Desilication of liquors in the Bayer industry has been examined fairly extensively by Addai-Mensah13,14 in several published papers. The treatment of the desilication data is presented in terms of crystal growth kinetics. This kinetic treatment utilizes a rate expression provided in Equation 4, where s is the supersaturation index, A is the total surface area for crystal growth, g is the order of crystal growth and kg is the growth rate constant.
-ds /dt = A kg s g Eq. 4
We examined the desilication data from Test 6 to derive the reaction order and the crystal growth rate. One obtains the reaction order by plotting the log (ds /dt) versus the log (s ) and the slope of the fitted line is the order of crystal growth, g. The intercept includes the growth rate constant and the surface area term. We were unable to deconvolute the surface area term from the growth rate constant.
In order to calculate ds /dt, a polynomial curve was fitted to a plot of the degree of supersaturation versus time. This plot is shown in Figure 4. The equation for this relationship gave a differential shown at Equation 5 where s is degree of supersaturation and t is time.
-ds /dt = 1.06 x 10-6 t2 – 3.06 x 10-4 t Eq. 5
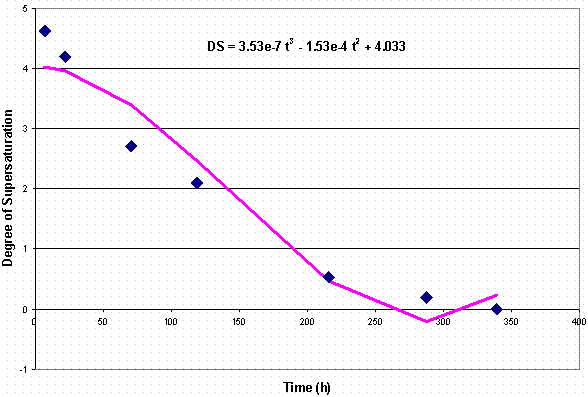
Figure 4. Plot of Degree of Supersaturation vs. Time
The estimate of the reaction order is shown in Figure 5. As mentioned previously, the slope of the relation between the log (ds /dt) and the log (s ) is the order of crystal growth. In studies of the Bayer process, the order of crystal growth has been measured14 between 1 and 3. As observed in Figure 5, the order of crystal growth in this Tank 38H simulant is 1.
Conclusions
In support of potential option for treating high level waste supernates that have elevated levels of silicon, a series of tests were performed to measure the desilication of these wastes as a function of time at an elevated temperature (50°C). These studies indicate that the addition of aluminate ion to typical wastes like Tank 38H supernate can reduce the soluble silicon concentration. Previous work in highly supersaturated simulants indicated reaction times would be on the order of days to weeks.
In this study, we examined simulants that were only mildly supersaturated with respect to aluminosilicate formation. Because the silicon concentration was limiting, the desilication data fit to pseudo first order reaction kinetic expressions. It was determined that the reaction rate constant was affected by the degree of supersaturation. This indicated that the reaction times to complete desilication of a high level waste supernate may be much longer than expected and may approach month-long time frames at 50°C. This option of reacting the soluble silicon by the addition of aluminate ion can be successful but will require longer than expected time.
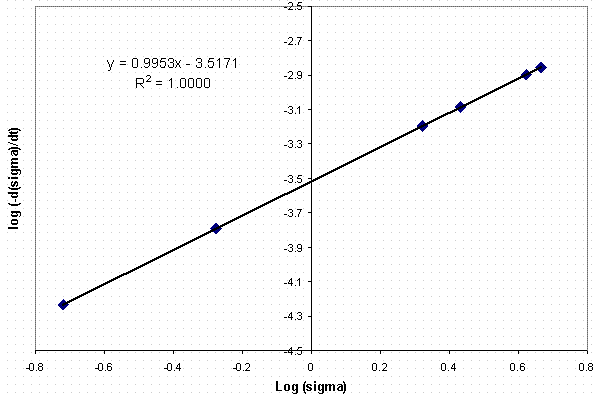
Figure 5. Plot of Log(-ds /dt) vs. Log (s )
References