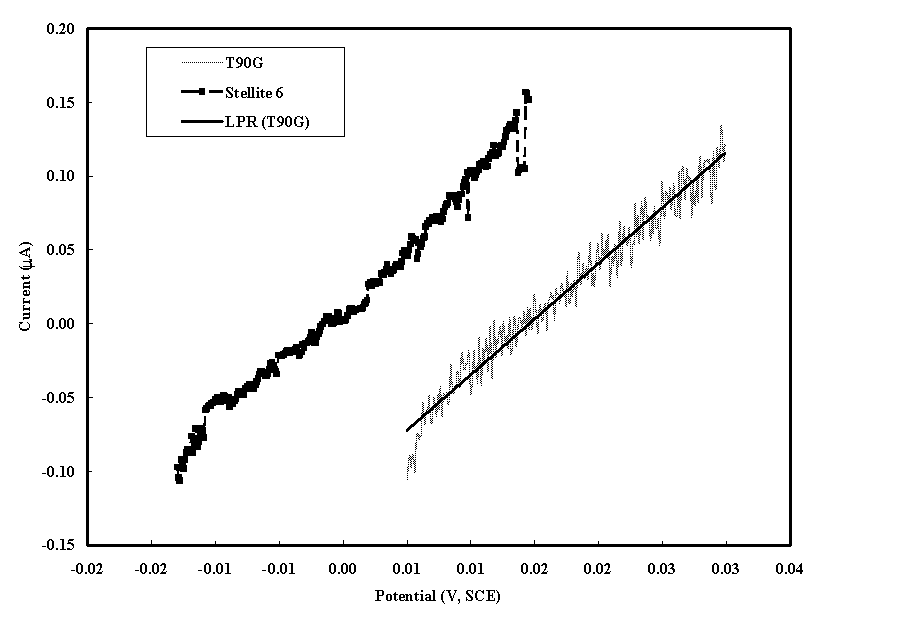
Figure 1. Linear Polarization Scans in Nitric Acid Solution (pH 2) at Room Temperature
WSRC-TR-2001-00445
Corrosion Assessment of T90G and Stellite 6 for DWPF
J. I. Mickalonis
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/index.html
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
1 Introductory Summary
The Savannah River Technology Center investigated the corrosion resistance of T90G a new iron-chromium alloy produce by GIW Industries, Inc (Augusta, GA). The material was being evaluated as a replacement material for the pump in the melter feed tank associated with the Defense Waste Processing Facility (DWPF). Electrochemical testing was performed at room temperature and 50 ° C in a nitric acid solution with a pH of two and in a simulated DWPF slurry. The performance of T90G was compared with Stellite 6, the current material of construction for the pump. Both materials displayed very low general corrosion rates (less than 1 mil per year (mpy)) and were not susceptible to pitting. A surface film was found to form at oxidizing potentials and did not appear to degrade the corrosion resistance.
2 Experimental Procedure
Electrochemical corrosion testing was performed to assess the corrosion resistance of T90G and Stellite 6. All tests were performed in accordance with the Savannah River Technology Center "Conduct of Research and Development: Integrated Safety Management for the R&D Environment" [1]. The applicable ASTM standards were used as guidelines for the testing [2,3]. All data was logged into Notebook WSRC-NB-99-00207 [4].
The electrochemical testing consisted of a sequential regime of potential monitoring (PM), linear polarization resistance (LPR), and potentiodynamic polarization (PDP). PM was performed during the initial stabilization after immersion of the test sample. The parameters for LPR included a scan rate of 0.167 mV/sec and a potential range of ± 15 mV from the open circuit potential (OCP). For PDP, the scanned potential (versus the OCP) was –100 mV to a vertex potential of 750 mV, then reversed to a final potential of 0 mV. The scan rate was also 0.167 mV/sec.
The test vessel was a five-port Greene-type electrochemical cell made of borosilicate glass. The counter electrodes were two graphite rods and the reference electrode was a saturated calomel electrode (SCE). The SCE was placed in a salt bridge, which contained 0.1 M sodium nitrate. Approximately 750 ml of solution was used for each test. The test vessel was heated on a standard laboratory hot plate with a temperature controller.
Table 1. Composition of Test Samples
|
Material |
Fe |
Cr |
Ni |
Mo |
Mn |
Co |
Other |
|
Stellite 6 |
2.3 |
27.2 |
2.1 |
0.2 |
-- |
60.5 |
5.46 W |
The test samples were cast rods of Stellite 6 (Deloro Stellite Company, Inc.) and T90G, a proprietary Fe/Cr/Mo alloy (GIW Industries, Inc.). The compositions of the alloys are shown in Table 1. These compositions were determined from x-ray fluorescence measurements. Test samples had a Teflonâ -coated copper wire attached with a conductive silver epoxy. The samples were mounted with a cold-set epoxy. The exposed surface was prepared for testing by grinding with a series of silicon carbon papers, ending with an 800-grit paper. The exposed surface had a surface area of approximately 2 cm2. Test samples were cleaned with ethyl alcohol and immediately tested.
The test solutions were either a nitric acid solution with a pH of 2 or a simulated DWPF slurry. The nitric acid solution was made with reagent grade concentrated nitric acid and distilled water. The slurry was a nitric acid based solution which contained various oxides. Table 2 shows the constituents that were in the solution. The slurry had a total solids content of 88.2%. The slurry pH was 4.25. The slurry was also analyzed by ion chromatograph for the anion content. Chlorides and sulfates, which are aggressive anions for corrosion, were approximately 40 and 400 ppm, respectively.
Table 2. DWPF Melter Feed Slurry Composition
|
Constituent |
Elemental (%) |
Oxide (%) |
||
|
Al |
10.6 |
19.9 |
||
|
Ca |
3.7 |
5.2 |
||
|
Cr |
0.2 |
0.3 |
||
|
Fe |
28.0 |
40.1 |
||
|
K |
0.2 |
0.2 |
||
|
Mg |
1.7 |
2.8 |
||
|
Mn
|
4.1
|
5.2
|
||
|
Na
|
8.2
|
11.1
|
||
|
Ni
|
0.3
|
0.4
|
||
|
Si
|
1.1
|
2.4
|
||
3 Corrosion Measurements
The test results from the electrochemical testing consisted of measurements of a linear polarization resistance for calculation of corrosion rate and polarization scans used to evaluate pitting susceptibility.
3.1 Linear Polarization Resistance
The linear polarization resistance (LPR) was measured from the applied potential and responding current. Figure 1 shows the data for both alloys in the pH 2 nitric acid solution at room temperature. In this potential region the current is linearly related to the potential. The LPR, which is shown for T90G in the plot, is the line slope. The corrosion rate (CR) is calculate from the LPR by the following equation [5]:
CR = B ´ EW / r ´ LPR . {1}
In this equation, the units for CR are mils per year (mpy), B is a constant which also contains conversion factors, r is the density (g/cm3), and EW is the equivalent weight. For CR units of mpy, the value of B is 0.1288 mpy-g/ m A-cm. The corrosion rates are shown in Table 3 for T90G and Stellite 6 in both the nitric acid solution and the simulated DWPF slurry. The electrochemical potentials (OCP) are also shown in the table. Data are given for each test performed.
Table 3. Corrosion Rates for T90G and Stellite 6
|
Solution |
T90G |
Stellite 6 |
||
|
CR (mpy) |
OCP (V, SCE) |
CR (mpy) |
OCP (V, SCE) |
|
|
Nitric at pH 2 |
||||
|
RT* |
0.06 |
0.015 |
ND |
ND |
|
DWPF Slurry |
||||
|
RT |
0.025 |
0.050 |
0.04 |
0.002 |
|
0.020 |
0.020 |
ND |
ND |
|
|
0.145 |
0.030 |
ND |
ND |
|
|
50 ° C |
0.028 |
0.101 |
0.018 |
0.070 |
|
0.014 |
0.090 |
0.030 |
0.067 |
|
* RT – room temperature
The corrosion rates of T90G in both solutions were extremely low, generally less than 0.1 mils per year, and were similar to those for Stellite 6, the current material of construction for the pumps. The room temperature data in the DWPF slurry shows the variability in corrosion rate that can occur between samples. The first two entries in the table for these conditions were performed on the same sample, while the third was performed with a different sample. This type of variability is not atypical for corrosion testing and at these corrosion rates is not significant for the application.
3.2 Polarization Scans
The corrosion characteristics of T90G showed passive behavior in both the nitric acid and DWPF sludge. In Figure 2, a cyclic polarization scan is shown for T90G in nitric acid at room temperature. The smaller current on the reverse scan indicates that there is no tendency for pitting. In Figure 3, the scans for T90G and Stellite 6 are shown for the DWPF sludge. Each alloy shows the passive behavior with no indication of pitting.
In the DWPF sludge unlike the nitric acid solution, each alloy had a jump in current at a potential of approximately 0.650 V (SCE). This increase occurred during the formation of a black surface film on the alloys. Potentiostatic tests were performed at potentials slightly above and below 0.650 V (SCE). The film did not form at potentials below 0.650 V (SCE) and current values were similar to those observed in the polarization scan (10-5 A). Above this potential the film formed; again, at similar currents observed in the polarization scan (10-3 A). This film could be easily removed by wiping after the tests. Visually, there was no indication of pitting below the film. This potential increase was associated with the oxidation of components of the DWPF solution.
3.3 Post-test Evaluation
After testing, the both samples were investigated to determine the source of the film and to confirm the macroscopic evaluation of the corroded surface.
An attempt was made to determine the chemical composition of the films even though they were thin. This attempt was to check if any of the constituents in the DWPF slurry were involved in the film. Since auger electron spectroscopy was unavailable, a scanning electron microscope equipped with x-ray fluorescence was used. The elemental results showed the presence of carbon, oxygen, and the constituents in the base materials only. The carbon and oxygen could be from the organics in the slurry.
After testing the samples were removed from the mounts and mounted in cross section to investigate the corroded morphology of the material. Micrographs at 100x are shown in Figure 4 for each material. The surfaces show a smooth surface with no indication of pitting. Numerous voids were observed in the T90G material, which was consistent with the as-received, as-cast material.
4 Conclusions
The corrosion resistance of T90G was similar to that of Stellite 6 in a DWPF slurry. In this solution and a nitric acid solution (pH=2), T90G had a corrosion rate less than 0.1 mpy. Although a black film formed on the material at oxidizing potentials, T90G was not susceptible to pitting. From a corrosion standpoint, this material should perform comparably to Stellite 6 in the application of the DWPF pump in the melter feed tank.
5 Acknowledgements
Karen Hicks provided outstanding service in performing the electrochemical tests and metallographic preparation. Jack Durden provided the x-ray fluorescence results.
6 References
3. ASTM Standard G59-97, "Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements," Annual Book of ASTM Standards, American Society of Testing and Measurements, 2000.

Figure 1. Linear Polarization Scans in Nitric Acid Solution (pH 2) at Room Temperature
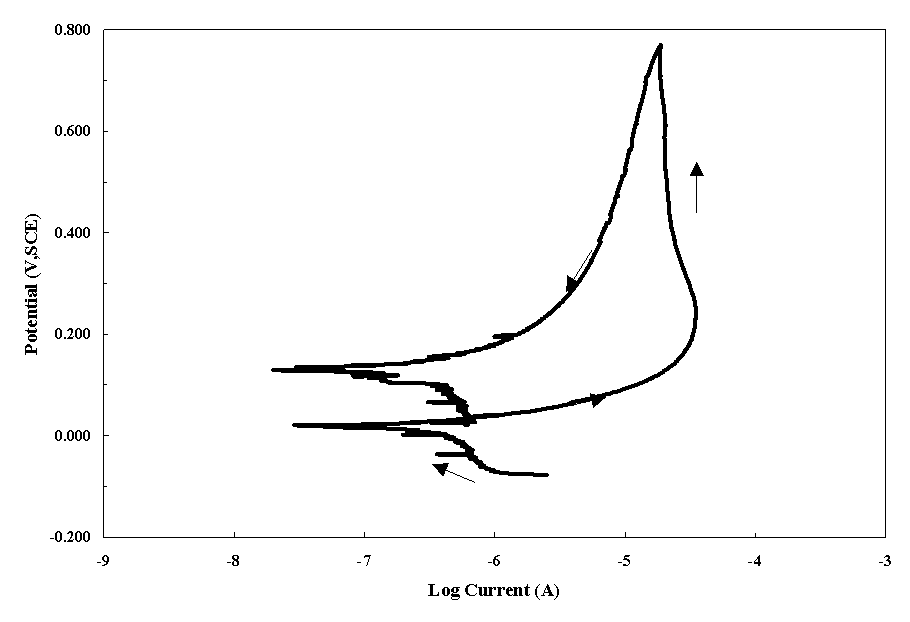
Figure 2. Cyclic Polarization Scan of T90G in Nitric Acid Solution (pH 2)
at Room Temperature
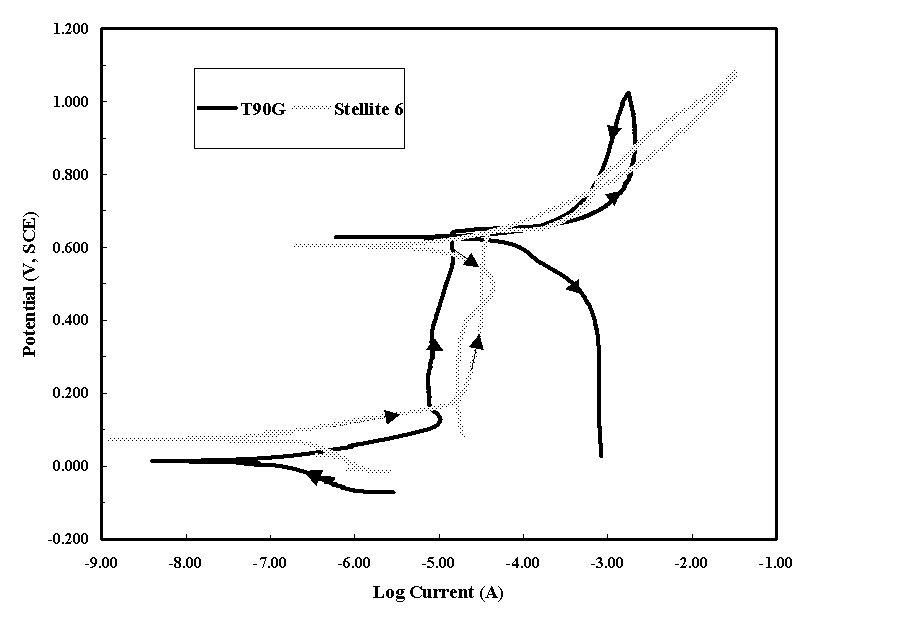
Figure 3. Cyclic Polarization Scans of T90G and Stellite 6 in Simulated DWPF Solution at Room Temperature