WSRC-TR-2000-00375
Proof-of-Concept of the Phytoimmobilization Technology
for TNX Outfall Delta: Status Report
Daniel I. Kaplan and Steven M. Serkiz
Westinghouse Savannah River Company
Aiken, SC 29808
Anna S. Knox and Thomas G. Hinton
Savannah River Ecology Laboratory
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Key Words: Phytoremediation, Ac, Co, Cr, Hg, Pb, Ra, Th, U, Stabilization, Wetlands, Apatite, Zeolite, Metallic Iron
1.0 Executive Summary
A series of proof-of-principle studies was initiated to evaluate the soil remediation technology, phytoimmobilization, for application at the TNX Outfall Delta (TNX OD) operable unit. Phytoimmobilization involves two steps. The first step is entitled phytoextraction, and it takes place mostly during the spring and summer. During this step the plants extract contaminants from the sediment into the roots and then translocate the contaminants to the above-ground plant parts. The second step is referred to as sequestration and it takes place largely during the autumn and winter when annual plants senesce or perennial trees drop their leafs. This step involves the immobilization of the contaminant once it leaches from the fallen leaf.
Leaf litter at the site was found to contain measurable concentrations of the constituents of concern (COCs; actinium, cobalt, chromium, mercury, lead, radium, thorium and uranium). Equally important, the leaf litter at the site was found to have a large annual biomass flux, 2000 kg/ha/yr. As part of a preliminary survey of the indigenous plants, it was discovered that a fern, Woodwardia areolata, contained exceptionally high contaminant concentrations. The species was particularly effective at accumulating cobalt and chromium. It also removed high concentrations of lead, uranium and thorium, and low concentrations of mercury. A greenhouse study is underway that will further quantify contaminant accumulations by the ferns.
In separate laboratory studies, sequestering agents were evaluated. Pyrite, a sulfide mineral, was found to have a distribution coefficient (Kd value) of 20,000 mL/g for mercury. Hydroxyapatite, a phosphate source, was able to remove large amounts of cobalt (Kd = 7700 mL/g), europium (an analogue for actinium; Kd = 720,000), lead (Kd = 138,000 mL/g), and uranium (Kd = 282,000 mL/g). Clinoptilolite, a zeolite cation exchange mineral, effectively removed barium (an analogue for radium; Kd = 6200 mL/g). A field demonstration of the various sequestering agent has been set up at the TNX OD.
The manner in which phytoimmobilization might be deployed at the site is to use the existing trees, which generates a lot of leaf-litter biomass with moderate contaminant concentrations, and to plant ferns, which generate less biomass, but greater contaminant concentrations. The sequestering agents should be made up of a combination of hydroxyapatite, clinoptilolite, and a sulfide source. Pieces of geomat, a layered material in which the sequestering agent is held between two geofabrics, should be used to emplacement the sequestering agents around the existing trees.
This proposed approach to remediating the ecologically sensitive wetland has a number of attributes. First, this approach uses existing natural geocycling processes and simply interrupts these processes by accumulating the contaminants in the geomat. Secondly, this approach should have minimal impact on the ecologically sensitive wetland at the TNX OD site; the geomat may be removed or left in place, depending on the risk. Finally, the technology should greatly reduce the cost of waste disposal by creating a concentrated waste in the sequestering agent.
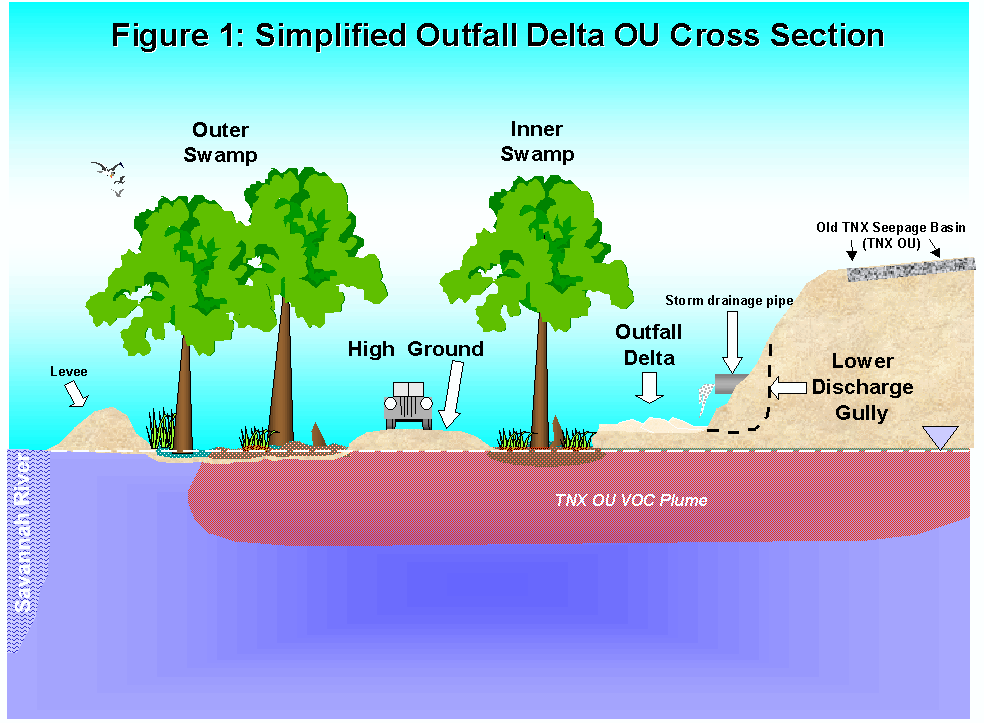
2.0 IntroductionThe TNX pilot-scale research facility located on the Savannah River Site, released process waste into an unlined seepage basin between 1958 and 1980. The basin, referred to as the Old TNX Seepage Basin, was designed to contain wastewater until it could seep into the underlying sediments, which would then act as an ion exchange media. The waste discharged to the Old TNX Seepage Basin included large quantities of chromium, mercury, sodium compounds, depleted uranium, thorium, and other radionuclides and heavy metals. The basin contents are believed to have entered the nearby inner and outer swamps by subsurface flow and overland flow; a result of purposely breaching a basin wall and routinely overfilling the basin (Figure 1).
The contaminants of concern at the operable unit include Ac, Co, Cr, Hg, Pb, Ra, Th and U. These contaminants are concentrated primarily in the upper 30-cm of sediment in the Inner Swamp area (WSRC 1999). A large portion of the operable unit is designated as a wetland. As such, the approaches applicable to remediating the site are limited due to the ecologically sensitive nature of the site. Among the most promising approaches to remediating the site are: soil mixing, which involves mixing a sequestering agent into the contaminated sediment, natural attenuation, which involves monitoring that the contaminants do not move off site, and phytoremediation, a broad term referring to any form of remediation in which plants are involved.
The subject of this report is the evaluation of a new form of phytoremediation referred to as phytoimmobilization. Phytoimmobilization involves two steps. The first step is entitled phytoextraction, and it takes place mostly during the spring and the summer. During this step the plants extract contaminants from the sediment into the roots and then translocate the contaminants to the aboveground plant parts (Figure 2). The second step is referred to as sequestration and it takes place largely during the autumn and winter (Figure 3). This involves the immobilization of the contaminant once it leaches from the fallen leaf or senescent plant during the autumn.

Figure 1. Simplified Outfall Delta Operable Unit Cross Section.
The sequestering agent used in this technology should have a number of qualities; it should:
The sequestering agent can be emplaced by mixing into the surface sediment, or as a geomat (Figure 4). A geomat consists of a sequestering agent placed in between two sheets of a geotextile. The advantage of the geomat configuration is that it can be removed. The advantage of the soil-mixing configuration is that no labor is required to make and remove the geomat. The question of which configuration to use is dependent on a number of issues, including the end use of the contaminated site and the risk associated with leaving the sequestered contaminants in place.
There are a number of different materials that can be used as sequestering agents (reviewed by Cantrell and Kaplan 1998). The criteria for selecting a sequestering agent will depend on the contaminants and the chemical composition of the background solution; in this case the background solution is plant leachate. Examples of sequestering agents include apatite for lead, cadmium, thorium and uranium, and sulfide minerals for mercury, silver, and tin.
The two most important attributes of phytoimmobilization are that it has minimal environmental impact on the site, and that it concentrates the waste. This latter point is especially important when compared to conventional phytoextraction approaches to remediating sites contaminated with radioactivity. Phytoextraction generates a large volume and mass of waste. There are few facilities that will incinerate radioactive waste. Thus, the radioactive waste removed from a site must be disposed of via subsurface burial. This is extremely costly, $80 per ft3 for mixed waste (personal communication with Bob Blundy, ERD).
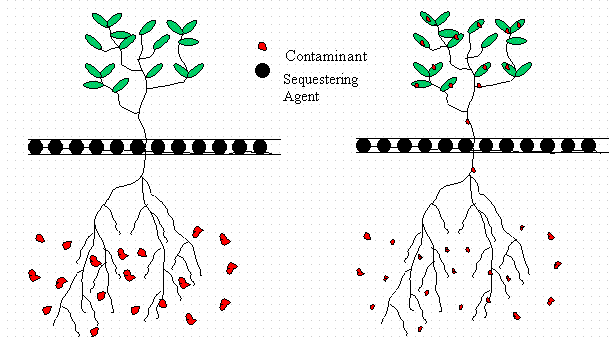
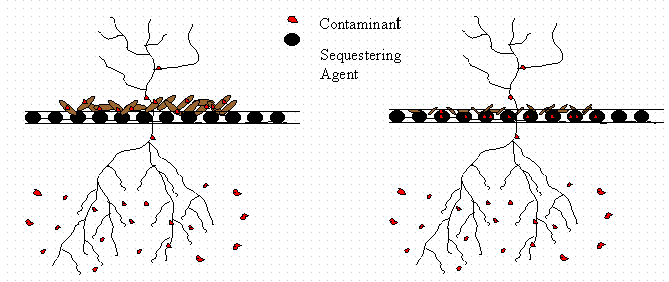
Figure 3. Second step in phytoimmobilization,
sequestration, involves immobilizing
the contaminants leached from aging plant material within a sequestering agent.

Figure 4. Emplacement of the sequestering
agent can be either (A), incorporated
into the contaminated sediment surface,
or (B) in the geomat configuration.
2.2 Objectives
The objectives of this study were to 1) conduct a proof-of concept of the phytoimmobilization technology, and 2) parameterize a computational design tool that could be used in the future deployment of this technology. Rather than conduct a field demonstration that would be costly and contain an unacceptable amount of scientific uncertainty, it was decided between SRTC and ER personnel to conduct a series of small experiments that would independently investigate each of the various processes involved in phytoimmobilization. The important advantage of this approach is that it permitted a large number of controlled experiments to be conducted that could evaluate and quantify the various processes that occur during deployment of the technology.
These experiments were organized to supply information that could be applied to a computational design tool, a linear kinetic reservoir model. The linear kinetic reservoir model (Lasaga 1980) uses matrix algebra to evaluate the concentration of a contaminant in various reservoirs as a function of time. The six reservoirs that were considered in the phytoimmobilization project are schematically presented in Figure 5. An example of the output from the linear kinetic reservoir model is presented in Figure 6. Contaminant concentrations on the sediments are in a steady state with the contaminant concentrations in the sediment pore water. Plants then take up the contaminants during the spring or summer. The leaves fall onto the sequestering agent during the autumn. The contaminants then leach out of the leaves into the underlying sequestering agent. The contaminants in the sequestering agent then come into steady state with the sediment water. During actual modeling, the water reservoirs before and after entering the sediment were combined due to their ephemeral nature and the difficulty of distinguishing them experimentally. Additionally, many of the input parameters used in the model are equilibrium constructs, such as sediment or sequestering agent Kd values. These constructs are converted into kinetic terms, i.e., rate terms that change contaminant concentration as a function of time, by making some assumptions about water flux. These important assumptions have not been entirely defined yet.
The various experiments that were conducted and how they are related to the conceptual model of the phytoimmobilization process are presented in Figure 7. These studies are:
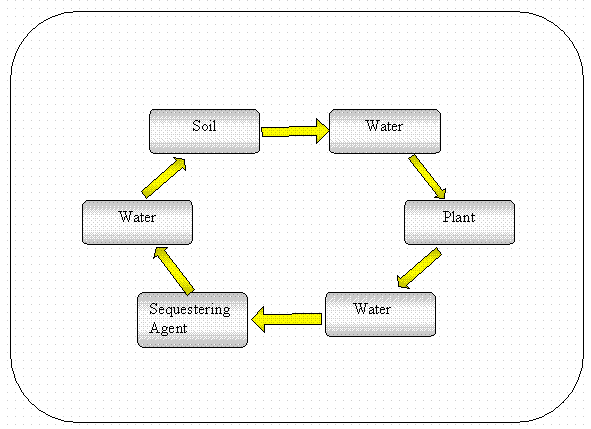
Figure 5. Contaminant reservoirs included in the linear kinetic reservoir model.
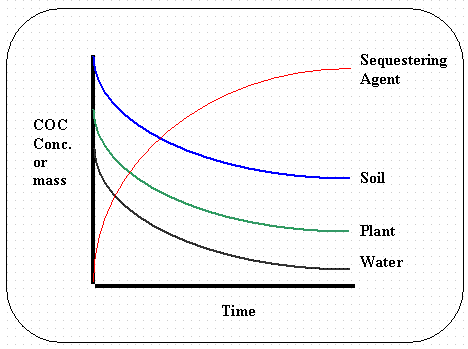
Figure 6. Example of the type of output that
the linear kinetic reservoir
model produces (COC stands for constituent of concern).
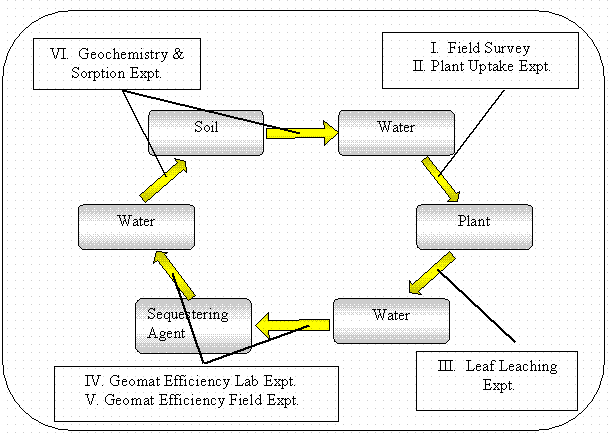
Figure 7. Experiments conducted and their relation to the linear kinetic reservoir model.
2.3 Scope
The constituents of concern (COCs) in these studies were Ac, Ba, Co, Cr, Hg, Pb, Ra, Th, and U. Evaluation of the technology was to be made relevant to the TNX OD operable unit. To reduce analytical cost, it was decided by SRTC and ER personnel to restrict analyses to ICP-MS and cold-vapor fluorescence spectroscopy, avoiding costly radiochemical analyses. Actinium and radium concentrations were below detection by the ICP-MS. Thus, Eu and Ce, both trivalent cations, were used as analogues for Ac biogeochemical behavior, and Ba, a divalent cation, was used as an analogue for Ra biogeochemical behavior. Europium, Ce, and Ra were easily detected by the ICP-MS. The cold-vapor fluorescence spectroscopy was used for Hg analyses.
2.4 Status
The project was broken down into 7 tasks, the six experiments described in the Objective section and a Modeling task. The status of each task is presented in Table 1.
Unexpected delays in the project occurred as a result of the August breakdown of the ICP-MS instruments at SREL and SRTC. A second and final version of this document will be issued 11/30/00 that will include the remaining data not reported in this first version.
Table 1. Status of Project Tasks.
|
Task |
Subtask |
Completion Date |
|
Field Survey of Plant and Soil Contaminant Concentrations |
Collect samples and extract soil and digest plant material |
Completed |
|
Analyze samples |
9/22/00 |
|
|
Plant Uptake Experiment |
Set up greenhouse experiment |
Completed |
|
Conduct first of 2 plant and soil samplings; extract/digest samples |
Completed(a) |
|
|
Analyze 1st plant and soil samples |
9/29/00 |
|
|
Leaf Leaching Experiment |
Set up experiment and collect samples as a function of time |
Completed |
|
Analyze samples |
9/29/00 |
|
|
Geomat Efficiency Laboratory Experiment |
Conduct Experiment |
Completed |
|
Analyze samples |
Completed |
|
|
Geomat Efficiency Field Experiment |
Set up experiment in field(b) |
Completed |
|
Geochemistry & Sorption Experiment |
Conduction sequential extractions |
Completed(c) |
|
Linear Kinetic Reservoir Model |
Write and test code |
Completed |
|
Run simulations |
10/16/00(d) |
|
|
(a) Second sampling is planned to be conducted next spring. (b) Sampling and chemical analyses is planned to be completed early next summer. (c) Laboratory portion of this task was completed last year (Kaplan et al. 1999). (d) This date or two weeks after the final analyses are completed, which ever comes later. |
||
3.0 Materials And Methods
There were seven tasks in this project: the six experiments depicted in Figure 7 and the Linear Kinetic Reservoir Modeling. The Materials and Methods of each of these tasks will be described below.
3.1 Field Survey of Plant and Soil Contaminant Concentrations
The objectives of this study were to determine, as a function of plant species, the contaminant concentrations and the concentration ratios (concentration in plant tissue divided by the concentration in the soil) in herbaceous plants and tree leaves of plants growing in the TNX OD site. A secondary objective was to determine the annual biomass flux (kg/m2/yr). A detailed description of the QA/QC and the sampling protocol used in this task is presented in the "Sampling and Analyses Plan for the Phytostabilization Study at the TNX Outfall Delta, Lower Discharge Gully and Swamp Operable Unit" (Kaplan 1999).
To accomplish the first objective, herbaceous plant, leaf litter, and soil samples were collected from 18 locations in the TNX OD (Figure 8). Three factors were considered when locating where to collect samples: 1) soil contaminant concentration, 2) number of soil contaminants present, and 3) soil type. The first two factors were evaluated by consulting with Environmental Restoration Division personnel who are familiar with the study site and by examining contaminant maps published in a recent report (WSRC 1999). The third factor, soil type, was identified from soil maps (basically, there is a wetland and a non-wetland, or upland, soil type in the TNX OD). Sample sites were located in both soil types and in areas containing the maximum number of contaminants at concentrations that could be readily detected. Additionally, two sample sites were located in uncontaminated areas near the TNX OD.
A soil, leaf litter and herbaceous plant sample was collected at each sample site during the week of December 11, 1999. The soil was collected by hand auguring down to 0.3-m. This upper portion of the soil profile was selected for sampling because it has been found to generally contain the highest contaminant concentrations (WSRC 1999). Leaf litter samples were collected from litter baskets located at the sample site, which are described in more detail below. Herbaceous samples were collected by cutting with gardening shears 5-cm from the ground. A dominant herbaceous species at each sample site was sampled. About 200-g of fresh leaf litter and herbaceous plant materials and 500-g soil were collected.
The soil was digested and extracted with 0.05 M DTPA. The DTPA extract procedure was taken from the agricultural literature and is an index of transition metal availability to plants.
A subset of the leaf litter samples was sorted by species, digested, and then analyzed for Eu, Co, Cr, Pb, Ba, Th, and U by ICP-MS, and for Hg by cold-vapor atomic fluorescence spectroscopy. All the herbaceous samples were digested and then analyzed for contaminant concentrations.
To accomplish the second objective, 40 0.5-m2 litter baskets were placed at the TNX OD site: 36 litter baskets were placed in the contaminated area, and 6 litter baskets were placed in nearby uncontaminated areas (Figure 9). The litter baskets were commercially available, plastic laundry baskets. They were suspended ~0.2-m above the ground with four PVC legs attached to the baskets. The litter baskets were randomly placed in the most contaminated area of the TNX OD in a statistical manner that followed a randomized split-plot experimental design; the split-plots were assigned to soil type. Of the 40 litter baskets, 14 were placed in the upland soil type and 26 were placed in the wetland soil type. The leaf litter was periodically collected during the fall. The leaves were brought back to the lab, dried and then weighed. A subset of the leaf litter samples was sorted by species, digested, and then analyzed for contaminant concentrations by ICP-MS for Eu, Co, Cr, Pb, Ba, Th, and U, and by cold-vapor atomic fluorescence spectroscopy for Hg.
Figure 8. Plant tissue and soil sampling locations at the
TNX OD.
Figure 9. Litter Basket locations at the TNX-OD.
3.2 Plant Uptake Experiment
The objective of this greenhouse study was to determine the contaminant concentrations that would accumulate in the above ground portion of ferns (Woodwardia areolata) and Bermuda grass grown in TNX OD contaminated soils. This study was conducted at the Savannah River Ecology Laboratory’s greenhouse facility. There was one uncontaminated and two contaminated soils (collected from coordinates B-5 and C-5 Figure 8). The fern was selected because, during a preliminary sampling, it was discovered that this plant species accumulated large concentrations of several of the targeted contaminants. The Bermuda grass was selected because its seeds are readily available, it will grow well in a wide range of growing conditions, and it is a monocot that may provide interesting insight into the mechanism of plant uptake.
The experimental factorial was 3 soil types, 2 plants, 4 replicates, and 2 fertilizer regimes. The two fertilizer regimes were with and without 25-kg/ha 10-10-10, N-P-K fertilizer added. In addition to these treatments there were 8 controls without plants: 2 fertilizer regimes x 2 soil types x 2 replicates. The uncontaminated soil provided a negative control. Two-kg of soil was added to each pot. Each soil-containing pot had one large hole on the bottom to permit excess water to leave the root zone. Each pot was placed in a larger pot without holes to contain contaminated water. Ferns were collected from a non-contaminated portion of the TNX OD and transplanted into the pots. The Bermuda grass was seeded directly into the pots. The plants were watered every work day and the water that would collect in the outside pot would be reintroduced to the soil each work day.
For the first sampling, approximately 50-g (wet) of plant material was collected. The sample was digested and then the samples were analyzed for contaminant concentrations. A second sampling is anticipated in early spring next year.
3.3 Leaf Leaching Experiment
The objective of this experiment was to determine the rate that contaminants leached from leaves. The plant material used in this study was a fern collected from an uncontaminated area of the TNX OD and from coordinate A-5 (Figure 9), within the contaminated area. Five grams of each fern material were placed in separate dialysis bags and then placed in 200-mL of uncontaminated surface water collected from near the TNX-OD. These tests were conducted in duplicate. This set up was placed in a platform shaker and sampled periodically over a two-month period. The 10-ml aliquots were then submitted for analyses by ICP-MS and cold-vapor atomic fluorescence spectroscopy.
3.4 Geomat Efficiency Laboratory Experiment
The objective of this laboratory study was to conduct a survey of potential sequestering agent materials. The following sequestering agents were tested:
Due to the different geochemistry of the studied elements, three separate laboratory experiments were conducted: a Cr experiment; a Hg experiment; and a Ba (used as an analog for radium), Co, Pb, and U experiment. All experiments were conducted in centrifuge tubes for a period of one week. Each treatment had three replicates. All three experiments were conducted with two background solutions: distilled water and rainwater with dissolved organic carbon (decomposed leaf-litter). Rainwater was collected in Aiken, SC, and leaf-litter was collected at a non-contaminated area of TNX. Approximately, 0.3-g of each sequestering agent was shaken with 30-mL of spike solution. The spike solution contained 1-mg L-1 Cr(VI), 2-mg L-1 Hg(II), and 50-m g L-1 of Ba, Co, Pb and U. The aqueous phases were analyzed for Cr, Ba, Co, Pb, and U by ICP-MS and for Hg by Cold-Vapor Atomic Fluorescence Spectrometer. The partitioning of the metals to the various sequestering agents was quantified by a distribution coefficient, Kd.
3.5 Geomat Efficiency Field Experiment
The objective of this study was to field test some of the better sequestering agents identified during the laboratory trials described above in Section 3.4.
The study was established in coordinate H-5 at the test site (Figure 9). Twenty-nine mesocosms were set up (Table 2). The mesocosms were 30-cm high and had a diameter of 15-cm. They were cut from PVC tubing. Within each mesocosm, the following layers were placed, starting from the bottom: 2-cm of sand (to act as a spacer so that the geomat does not come into contact with the contaminated soil), and 1.5-cm geomat. The geomat was made by cutting 16-cm diameter circles out of a geofabric (AMOCO Style 5412, Atlanta, GA). The edges of two geofabric circles were sewed together, leaving a 3-cm opening. Through this opening 400-g of sequestering agent was added. The opening was then sewed together. A screen was placed on top of the mesocosm to minimize the occurrence of leaves falling into the mesocosm, but at the same time permitting rain to enter.
Ferns and leaf litter will be collected this autumn from coordinates A-5 and B-5. Each material will be homogenized, ground into ~1-cm pieces, and then added to the mesocosms. At this time (time = 0), a subsample of the leaves will be analyzed for contaminant concentrations.
After 3 months, a subsample of the leaves and geomat material will be digested and analyzed for contaminant concentrations. Also at this time, it will be determined whether to continue the study as is, or to remove the remaining plant tissue and add new fresh material from the same homogenized sample used at the start of the experiment. If a great deal leaf decay has taken place, than the 9old leaves will be removed and more fresh leaves will be added.
Because the biomass flux will be known for both the leaf litter and the fern, the amount of plant material added to the mesocosms will be translated into terms of "number of years worth of plant material." This arrangement will permit us to evaluate tens of years worth of leaf litter in one year.
Table 2. Experimental matrix for the Geomat Efficiency Field Experiment.
|
Treatment |
# Replicates |
Leaf Material |
|
|
1 |
Fe(0) |
3 |
Leaf Litter |
|
2 |
Apatite |
3 |
Leaf Litter |
|
3 |
Clinoptilolite |
3 |
Leaf Litter |
|
4 |
Clinoptilolite/Apatite/Fe(0) |
3 |
Leaf Litter |
|
5 |
Fe(0) |
2 |
Fern |
|
6 |
Apatite |
2 |
Fern |
|
7 |
Clinoptilolite |
2 |
Fern |
|
8 |
Clinoptilolite/Apatite/Fe(0) |
2 |
Fern |
|
9 |
Fe(0) |
3 |
None |
|
10 |
Apatite |
3 |
None |
|
11 |
Clinoptilolite |
3 |
None |
3.6 Geochemistry & Sorption Experiment
The objective of this study was to determine the relative availability of the various contaminants and to determine distribution coefficients, Kd values, that could be used in the Linear Kinetic Reservoir Model. The laboratory portion of this work was completed as part of a previous study (Kaplan et al. 1999). The site-specific field data taken from this report and that will be used in our modeling is presented below as part of the Results and Discussion of the Linear Kinetic Reservoir Model.
3.7 Linear Kinetic Reservoir Model
The linear kinetic reservoir model is based on the well-established geocycling model that was first proposed by Lasaga (1980). Computer code to execute the model has been written and preliminary input values have been selected. These input values are presented in the Results and Discussion Section. A full description of the application of this model for describing the phytoimmobilization at the TNX OD will be included in the final report.
4.0 Results And Discussion
4.1 Field Survey of Plant and Soil Contaminant Concentrations
Sixteen sets of soil, herbaceous, and leaf litter samples were collected for this survey. Plant samples were totally digested. It was important to collect soil samples along with the plant samples because, low concentrations in the plant tissue could be attributed to either low soil contaminant concentrations or low plant uptake rates. The soils were digested to provide a measure of the total amount of each contaminant in the soil and were also extracted with a DTPA solution to provide an index of the plant available fraction. For phytoimmobilization, a measure of the plant available fraction in the soil is important and likely represents only a fraction of the total. The percentage of DTPA extractable compared to the total fraction will likely vary with element. Strongly sorbing contaminants, such as lead and mercury, are likely to have appreciably less DTPA extractable concentrations than total concentration. Conversely, chromium, a weakly sorbing anion, is likely to have a relatively large DTPA-extractable fraction.
As discussed in the Status section, very little of the analytical results from the field survey data have been completed. All the analytical data for the total digestion and DTPA extraction is completed but was delivered to us too late to be included in this report. The biomass annual flux data and some plant and soil analytical results, albeit quite limited, have been completed and will be presented here.
In the first stage of phytoimmobilization, phytoextraction, herbaceous and woody plants are used to remove contaminants from soil. In this technology, plants are not harvested but fall and decompose on the sequestering agent. In this study, the main focus was on evaluating the ability of indigenous plants to take-up soil contaminants. This study permitted us to screen plant species for more in-depth future studies.
4.1.1 Soil Properties at the TNX OD
Selected properties of an uncontaminated soil collected from coordinate BGCH05 and a contaminated soil collected from coordinate A-5 are presented in Table 3. Both soils are acidic, contain moderate levels of organic matter and have a sandy texture. The cation exchange capacity (CEC) values are typical of this area. Also, reported in Table 3 are anion exchange capacity (AEC) values. This parameter is like CEC except it is for anions, and it has been shown to increase substantially in SRS soils under increasingly acidic conditions (Kaplan et al. 2000). More detailed properties and the geochemistry of the TNX OD soil are reported by Kaplan and Serkiz (1999).
Contaminant concentrations in soils are presented in Table 4. Concentrations of Ag, Cr, Co, Cu, Hg, Pb, Th, and U-238 are appreciably greater in the A-5 soil than in the background soil. For U-238, there is a four order-of-magnitude difference between the concentrations in these two soils. We are not interested in the concentration of Ba and Ce per se, but are interested in these elements only in so far that they can be used as analogues for Ra and Ac, respectively.
Table 3. Soil characterization
of an uncontaminated background soil
collected from coordinate BGCH05 and a
contaminated soil collected from coordinate A-5.
|
Soil |
>2-mm |
Sand |
Silt |
Clay |
pH |
Organic C |
CEC |
AEC |
Fe-oxides(b) |
|
(%,wt) |
(%,wt) |
(%,wt) |
(%,wt) |
(mg/kg) |
(cmol(+) /kg) |
(cmol(-) /kg) |
(%,wt) |
||
|
Uncontaminated Background |
0.8 ± 1.0 |
79.4 ± 2.1 |
13.6 ± 0.3 |
6.3 ± 0.8 |
4.16 ± 0.01 |
1395 |
4.75 ± 0.08 |
1.56 ± 0.17 |
0.01 |
|
Contaminated A-5 |
21.2 ± 6.3 |
48.8 ± 6.8 |
23.6 ± 1.6 |
6.4 ± 1.1 |
4.00± 0.08 |
1493 |
8.96± 0.09 |
2.43± 0.05 |
0.08 |
|
(a) Analyses were conducted as duplicates or without duplication (where no standard deviation is presented). (b) Fe-oxides: extracted by sodium dithionite from total soil (an estimate of concentration of Fe-oxide coatings); reported as % Fe2O3. |
|||||||||
Table 4. Elemental
Composition (m g/g) of an uncontaminated background
soil
collected from coordinate BGCH05 and a contaminated soil collected from
coordinate A-5.
|
Soil |
Ag |
Al |
As |
Ba |
Be |
Cd |
Ce |
Cr |
Co |
Cu |
|
Uncontaminated Background |
<0.0001 |
1915 |
0.36 |
21.97 |
0.46 |
<0.0002 |
19.40 |
2.82 |
0.99 |
30.04 |
|
Contaminated A-5 |
1.8(a) |
6252 |
0.57 |
78.72 |
0.80 |
0.33 |
53.56 |
44.60 |
3.69 |
88.25 |
|
Fe |
Hg |
Mn |
Ni |
Pb |
Sr |
Tl |
Th-232 |
U-235 |
U-238 |
|
|
Uncontaminated Background |
2635 |
0.034 |
84.28 |
1.87 |
4.09 |
1.46 |
0.11 |
2.71 |
<0.002 |
0.6 |
|
Contaminated A-5 |
7533 |
6.821 |
114.37 |
18.82 |
17.60 |
7.16 |
0.08 |
201.01 |
1.20 |
187.7 |
4.1.2 Annual Biomass Flux Estimates
Table 5 contains the mass of leaf litter collected from 40 sampling baskets located in the contaminated area and 6 sampling baskets located in the uncontaminated area of the study site. However, the sampling baskets were placed after the deciduous leaves at the site had started to fall, consequently, they do not provide a measure of the annual leaf fall. Due to radiological safety concerns, we were not permitted to collect the leaves beneath the baskets that had already fallen in the contaminated area. However, we were able to collect and weigh the leaves beneath the collection baskets located in the uncontaminated area; this permitted calculation of the annual biomass flux (Table 6). Based on these 6 samples, the annual biomass flux is:
Only 24% and 33% of the total leaf litter that fell during the fall of 1999 was collected in the baskets located in the wetlands and uplands, respectively. For the fall of 2000, all 46 baskets will be in place at the start of the leaf-falling season and should provide additional data to estimate the biomass annual flux.
Table 5. Leaf litter mass collected on March
15, 2000 from sampling baskets placed in
40 locations in the contaminated area
and 6 locations in the uncontaminated area.
|
Site Coordinate |
Contamination/Control |
Soil Type |
Leaf Litter Mass (g) |
|
A-2 |
Contaminated |
Wetland |
61.63 |
|
A-3 |
Contaminated |
Wetland |
51.2 |
|
A-4 |
Contaminated |
Wetland |
35.65 |
|
A-5 |
Contaminated |
Wetland |
33.18 |
|
A-7 |
Contaminated |
Wetland |
58.45 |
|
B-2 |
Contaminated |
Wetland |
89.25 |
|
B-3 |
Contaminated |
Wetland |
55.93 |
|
B-4 |
Contaminated |
Wetland |
46.67 |
|
B-5 |
Contaminated |
Wetland |
22.97 |
|
B-6 |
Contaminated |
Wetland |
51.29 |
|
B-7 |
Contaminated |
Wetland |
50.82 |
|
B-8 |
Contaminated |
Wetland |
54.83 |
|
C-2 |
Contaminated |
Upland |
73.32 |
|
C-3 |
Contaminated |
Upland |
52.6 |
|
C-4 |
Contaminated |
Upland |
50 |
|
C-5 |
Contaminated |
Upland |
39.14 |
|
C-7 |
Contaminated |
Wetland |
51.38 |
|
C-8 |
Contaminated |
Wetland |
51.3 |
|
D-3 |
Contaminated |
Upland |
44.2 |
|
D-4 |
Contaminated |
Upland |
47.8 |
|
D-5 |
Contaminated |
Upland |
39.1 |
|
D-6 |
Contaminated |
Wetland |
32.1 |
|
D-7 |
Contaminated |
Wetland |
38.9 |
|
D-8 |
Contaminated |
Wetland |
39.32 |
|
G-1 |
Contaminated |
Wetland |
84.1 |
|
G-11 |
Contaminated |
Wetland |
79.32 |
|
G-3 |
Contaminated |
Wetland |
61.65 |
|
G-7 |
Contaminated |
Wetland |
52.49 |
|
H-1 |
Contaminated |
Wetland |
57.33 |
|
H-2 |
Contaminated |
Wetland |
92.77 |
|
H-4 |
Contaminated |
Upland |
53.85 |
|
H-5 |
Contaminated |
Upland |
54 |
|
H-6 |
Contaminated |
Upland |
81.8 |
|
H-7 |
Contaminated |
Upland |
62.64 |
|
C-18 |
Contaminated |
Upland |
53.89 |
|
C-17 |
Contaminated |
Upland |
76.46 |
|
D-17 |
Contaminated |
Upland |
44.84 |
|
I-17 |
Contaminated |
Wetland |
39.86 |
|
I-18 |
Contaminated |
Wetland |
60.19 |
|
J-17 |
Contaminated |
Wetland |
78.5 |
|
TNXOFD-BG1 |
Control |
Wetland |
50.78 |
|
BGCH-7 |
Control |
Wetland |
21.32 |
|
K-26 |
Control |
Wetland |
24 |
|
BGTRO3 |
Control |
Upland |
24.3 |
|
West of 44 (50m) |
Control |
Upland |
27.4 |
|
East of 44 (50m) |
Control |
Upland |
46.82 |
Table 6. Annual biomass flux estimates for leaves at the TNX OD.(a)
|
Soil Type |
Sample ID |
Leaf Litter in Basket (g/0.5 m2) |
Leaf Litter Beneath Basket (g/0.5 m2) |
Total Leaf Litter |
Mass(b) |
Mass(b) (kg/ha/yr) |
% in Basket |
Ave. % in Basket |
|
Wetland |
TNXOFD-BG1 |
50.78 |
97.0 |
147.78 |
133 ± 15 |
2669 ± 296 |
34 |
24 ± 10 |
|
BGCH-7 |
21.32 |
113.04 |
134.36 |
- |
- |
16 |
- |
|
|
K-26 |
24 |
94.2 |
118.2 |
- |
- |
20 |
- |
|
|
Upland |
BGTRO3 |
24.3 |
54.3 |
78.6 |
100 ± 19 |
2000 ± 374 |
31 |
33 ± 8 |
|
West of 44 (50m) |
27.4 |
80.95 |
108.35 |
- |
- |
25 |
- |
|
|
East of 44 (50m) |
46.82 |
66.26 |
113.08 |
- |
- |
41 |
- |
|
|
(a) Leaf litter was collected between September 20, 1999 and March 15, 2000. By September 20, 1999, leaves had already started falling. The recently fallen leaves beneath the leaf litter baskets were collected and weighed and their mass is reported in "Leaf Litter Beneath Basket. " By March 15, 2000, essentially all of the deciduous leaves had fallen. The leaf litter baskets had an area of 0.5-m2. (b) Average ± standard deviation. |
||||||||
4.1.3 Plant Species Abundance
The material collected in 21 leaf litter baskets was separated by species; the percent of the mass of each species is presented in Table 7 and Table 8. A summary of the relative abundance of the dominant species is present in Table 9. Water oak, tupelo, baldcypress and loblolly pine account for 43% of the total leaf litter mass.
Table 7. Specie-composition of leaf litter (% g species/100 g total leaf litter) (Table continues in Table 8).(a)
|
Location Map Node |
Soil Type |
Cypress |
Willow |
Pine |
Sycamore |
Red Maple |
Unknown |
Water Oak |
Oak |
Tupelo |
Tupelo Seeds |
|
A-2 |
Wetland |
35 |
- |
11 |
- |
4 |
- |
1 |
2 |
44 |
- |
|
A-5 |
Wetland |
11 |
- |
3 |
- |
55 |
- |
21 |
- |
5 |
- |
|
A-7 |
Wetland |
25 |
- |
9 |
- |
4 |
- |
7 |
- |
35 |
17 |
|
B-2 |
Wetland |
41 |
- |
15 |
- |
7 |
- |
2 |
- |
28 |
- |
|
B-3 |
Wetland |
19 |
- |
- |
- |
15 |
- |
2 |
- |
55 |
- |
|
B-4 |
Wetland |
28 |
- |
- |
- |
3 |
- |
1 |
- |
4 |
- |
|
B-5 |
Wetland |
44 |
- |
- |
- |
56 |
- |
- |
- |
- |
- |
|
C-3 |
Upland |
17 |
- |
8 |
41 |
7 |
- |
- |
- |
6 |
- |
|
C-4 |
Upland |
5 |
15 |
23 |
31 |
19 |
2 |
4 |
- |
- |
- |
|
C-5 |
Upland |
3 |
- |
36 |
- |
52 |
5 |
- |
4 |
- |
- |
|
D-3 |
Upland |
2 |
- |
20 |
21 |
- |
- |
10 |
- |
- |
- |
|
D-6 |
Wetland |
- |
- |
- |
26 |
- |
- |
11 |
29 |
5 |
- |
|
D-7 |
Wetland |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
D-8 |
Wetland |
10 |
- |
26 |
- |
- |
- |
15 |
- |
47 |
- |
|
G-2 |
Wetland |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
G-3 |
Wetland |
37 |
- |
- |
- |
- |
- |
9 |
- |
22 |
12 |
|
G-7 |
Wetland |
- |
- |
36 |
- |
- |
- |
36 |
- |
- |
- |
|
TNXOFD-BG1 |
Wetland |
5 |
- |
60 |
- |
- |
- |
16 |
- |
- |
- |
|
BGCH-7 |
Wetland |
- |
- |
54 |
- |
- |
- |
46 |
- |
- |
- |
|
K-26 |
Wetland |
- |
- |
11 |
- |
9 |
- |
76 |
- |
- |
- |
|
BGTRO3 |
Upland |
- |
- |
6 |
- |
- |
9 |
75 |
- |
- |
- |
|
West of 44 (50m) |
Upland |
- |
- |
7 |
- |
- |
- |
8 |
- |
- |
- |
|
East of 44 (50m) |
Upland |
- |
- |
6 |
- |
- |
- |
75 |
- |
19 |
- |
|
|||||||||||
Table 8. Specie-composition of leaf litter (% g species/100 g total leaf litter) (Table continues in Table 7).(a)
|
Map Node Location |
Soil Type |
Sweetgum |
Iron Wood |
Vitis spp. |
Ilex opaca |
Sticks |
Beech |
Hickory |
Beauty Berry |
|
A-2 |
Wetland |
- |
- |
- |
- |
2 |
- |
- |
1 |
|
A-5 |
Wetland |
4 |
- |
- |
- |
- |
- |
- |
|
|
A-7 |
Wetland |
3 |
1 |
- |
- |
- |
- |
- |
- |
|
B-2 |
Wetland |
- |
- |
- |
- |
7 |
- |
- |
- |
|
B-3 |
Wetland |
2 |
- |
- |
- |
7 |
- |
- |
- |
|
B-4 |
Wetland |
45 |
- |
6 |
- |
12 |
- |
- |
- |
|
B-5 |
Wetland |
- |
- |
- |
- |
- |
- |
- |
- |
|
C-3 |
Upland |
12 |
- |
- |
- |
9 |
- |
- |
- |
|
C-4 |
Upland |
- |
- |
- |
- |
- |
- |
- |
- |
|
C-5 |
Upland |
- |
- |
- |
- |
- |
- |
- |
- |
|
D-3 |
Upland |
26 |
2 |
14 |
5 |
- |
- |
- |
- |
|
D-6 |
Wetland |
29 |
- |
- |
- |
- |
- |
- |
- |
|
D-7 |
Wetland |
- |
- |
- |
- |
- |
- |
- |
- |
|
D-8 |
Wetland |
- |
2 |
- |
- |
- |
- |
- |
- |
|
G-2 |
Wetland |
- |
- |
- |
- |
- |
- |
- |
- |
|
G-3 |
Wetland |
- |
- |
- |
- |
20 |
- |
- |
- |
|
G-7 |
Wetland |
10 |
9 |
- |
3 |
- |
- |
6 |
- |
|
TNXOFD-BG1 |
Wetland |
- |
- |
- |
6 |
11 |
3 |
- |
- |
|
BGCH-7 |
Wetland |
- |
- |
- |
- |
- |
- |
- |
- |
|
K-26 |
Wetland |
- |
4 |
- |
- |
- |
- |
- |
- |
|
BGTRO3 |
Upland |
- |
- |
- |
- |
- |
4 |
6 |
- |
|
West of 44 (50m) |
Upland |
30 |
- |
- |
- |
- |
55 |
- |
- |
|
East of 44 (50m) |
Upland |
- |
1 |
- |
- |
- |
- |
- |
- |
|
(a) Empty cells within the table indicate 0% of species was present in the sample. Total leaf litter mass is presented in Table 5. The scientific names for many of these plant species are presented in Table 9. |
|||||||||
Table 9. Mass of plant species collected from 21-leaf litter baskets located in the contaminated portion of the TNX OD.
|
Common Name |
Scientific Name |
Mass (kg/ha) |
% Mass |
|
Water Oak |
Quercus nigra |
98 |
11 |
|
Tupelo |
Nyssa sylvatica |
97 |
11 |
|
Baldcypress |
Taxodium distichum |
96 |
11 |
|
Loblolly Pine |
Pinus taeda |
89 |
10 |
|
Red Maple |
Acer rubrum |
52 |
6 |
|
Sweetgum |
Liquidambar styraciflua |
48 |
5 |
|
American Sycamore |
Platanus occidentalis |
29 |
3 |
|
Other |
381 |
43 |
|
|
Total biomass |
890 |
100 |
|
|
(a) Leaf litter was collected between September 20, 1999 and March 15, 2000. By September 15, 1999, leaves had already started falling. Thus these values do not represent a total leaf litter flux. The leaf litter baskets had an area of 0.5-m2. |
|||
4.1.4 Contaminant Uptake by Plants Growing in the TNX OD
As part of a preliminary study, five plants and unsorted leaf litter collected from coordinate A-5 were analyzed for targeted contaminant concentrations (Table 10). In addition to reporting contaminant concentrations in the plants, the data was normalized for differences in soil contaminant concentrations by calculating concentration ratios (CR = mg kg-1 dry plant / mg kg-1 dry soil). One herbaceous plant, a fern (Woodwardia areolata), had a high propensity to uptake all targeted contaminants, especially U, Th, and Hg. Also important to note is that the leaf litter contained appreciable concentrations of the targeted contaminants. This is notable because the biomass of the leaf litter is large in this area, and will always account for a majority of the annual biomass, even if a monoculture of a hyperaccumulater was to be introduced to the site for remediation purposes. The leaf litter had high concentration of Co (17 mg/kg) and also a high CR (4.59). The high Co concentration and CR in the leaf litter needs to be further evaluated.
Table 10. Elemental composition of plants collected from coordinate A-5 within the TNX OD on November 15, 1999.
|
Plant/Tree species |
Co |
Cr |
Hg
|
Pb |
U-238
|
Th-232 |
|||||||
|
mg/ |
CR |
mg/kg |
CR |
mg/ kg |
CR |
mg/ kg |
CR |
mg/ kg |
CR |
mg/kg |
CR |
||
|
Fern |
2.4 |
0.65 |
4.7 |
0.11 |
0.8 |
0.12 |
0.7 |
0.17 |
20.7 |
0.11 |
21.5 |
0.107 |
|
|
Grass |
0.8 |
0.10 |
1.7 |
0.04 |
BDL |
--- |
0.6 |
0.15 |
0.6 |
0.003 |
0.8 |
0.004 |
|
|
Redmaple |
0.3 |
0.08 |
1.0 |
0.02 |
0.1 |
0.01 |
0.3 |
0.07 |
0.3 |
0.002 |
0.3 |
0.002 |
|
|
Baldcypress |
0.4 |
0.11
|
0.8 |
0.02 |
0.0 |
0.00 |
0.2 |
0.05 |
0.2 |
0.001 |
0.3 |
0.002 |
|
|
Sweetgum |
0.5 |
0.14 |
1.0 |
0.02 |
0.1 |
0.01 |
0.2 |
0.05 |
4.0 |
0.021 |
0.3 |
0.002 |
|
|
Leaf-litter (b) |
17.0 |
4.59 |
1.1 |
0.02 |
BDL |
--- |
0.3 |
0.07 |
2.9 |
0.015 |
0.3 |
0.002 |
|
|
(a) CR is the concentration ratio of plant concentration divided by soil concentration (mg/L plant/ mg/L soil). (b) Includes many plant species. (c) B.D.L. = below detection limit, which is ~0.01 mg/L Hg. |
|||||||||||||
4.2 Plant Uptake Experiment
The Plant Uptake Experiment is a greenhouse study that is growing Bermuda grass and a fern, Woodwardia areolata, found in the preliminary field survey to take up high concentrations of the contaminants. The plants are growing in 2 soils collected from the TNX OD contaminated area. The first of two harvests has been completed. The second harvest will be conducted later next year. No analytical data is presently available.
4.3 Leaf Leaching Experiment
The Leaf Leaching Experiment is designed to quantify the rate that contaminants leach from various plant materials. The two plant materials being evaluated Woodwardia areolata and unsorted leaf litter, were both collected from coordinate A-5. The experiments were conducted in duplicate. No analytical data is presently available.
4.4 Geomat Efficiency Laboratory Experiment
In the second stage of phytoimmobilization, sequestration, contaminants from decomposed plant material would not partition directly to soil, but instead, sorb onto the sequestering agent, or geomat. Geomats are made from minerals, such as apatite, zeolite, metallic iron, and others. Ground-up minerals can be placed inside of geotextile material (e.g., AMOCO Style 5412).
Among the potential sequestering agents tested, only metallic iron was effective at removing Cr (VI) from solution (Table 11). Metallic iron removes soluble Cr (VI) from solution by converting the soluble Cr (VI) species to the sparingly soluble Cr (III) species (Cantrell et al. 1995). Thus, the removal of Cr (VI) from solution is not via adsorption, as the Kd construct implies; instead, it is by reductive precipitation (Equation 1).
CrO42- + 3/2Feo + 5H+ = Cr(OH)3 + H2O + 3/2Fe 2+ (1)
Mixtures of metallic iron with other sequestering agents were evaluated because it is likely that more than one material will be required to sequester all eight contaminants. The addition of North Carolina apatite, hydroxyapatite, and clinoptilolite (a type of zeolite) greatly decreased the Cr (VI)-removal effectiveness of the metallic iron. The pH of the equilibrium solutions at the end of the contact time between the sequestering agents and the aqueous Cr(VI) did not vary greatly between treatments. As expected, the pH of the metallic iron, Fe(0), treatment increased. This can be attributed to the metallic iron reducing water, thereby creating hydroxides as shown in Equation 2.
Fe0 + H2O = Fe2+ + OH- + 1/2H2(gas) (2)
Table 11. Chromium distribution coefficients (Kd) for several sequestering agents in a SRS surface water.
|
Treatment |
Avg. pH |
Avg. Equilibrium |
Kd |
|
SRS Blank Control |
4.6 |
0.02 |
-- |
|
Cr Spike Control |
4.4 |
1.00 |
-- |
|
Fe(0) |
5.8 |
0.26 |
294 ± 8 |
|
Fe(0) / NC Apatite |
5.7 |
0.46 |
66 ± 25 |
|
Fe(0) / Hydroxyapatite |
5.3 |
0.67 |
26 ± 4 |
|
Fe(0) / Clinoptilolite |
5.2 |
0.63 |
11 ± 1 |
|
Fe(0) / NC-Apatite / Clinoptilolite |
5.3 |
0.78 |
10 ± 2 |
|
Fe(0) / Hydroxyapatite / Clinoptilolite |
5.2 |
0.68 |
17 ± 0 |
|
(a) 0.3 g solid: 30-mL SRS surface water; 1-wk equilibration period; 3 replicates; total mass of mixtures = 0.3 g evenly divided between sequestering agents. |
|||
Evaluation of Hg sequestering agents was conducted in uncontaminated SRS surface water containing 6 mg/L total organic carbon, and a leaf litter leachate containing >100 mg/L total organic carbon. The Kd values in the presence of dissolved organic matter tended to be lower than when the organic matter was not present (Table 12). Thus, it appears the presence of the strongly complexing organic ligands present in plant leachate moderately decrease the removal effectiveness with these sequestering materials.
Pyrite had a Kd value ~ 20,000 mL/g and metallic iron had a Kd value of >1000 mL/g (Table 12). The reduction in soluble Hg concentrations in pyrite (FeS2) treated solutions is not surprising because the pyrite can release sulfides, which can then combine with Hg(II) to form the sparingly soluble mercury-sulfide species, cinnabar, HgS (Bodek et al. 1988). The relatively low Kd for the gypsum (CaSO4) addition may be attributed to the fact that the test suspension was oxic, therefore the sulfate in the gypsum was never reduced to sulfide, the form of sulfur that forms the sparingly soluble mercury precipitate. In a soil system where microbes are present and where reducing conditions exist, it is very likely that gypsum will be a better sequestering agent for mercury. Thus, evaluation of gypsum for this application needs to be conducted under conditions more similar to those of its final application, namely in the presence of soils that may convert the sulfate to sulfide.
The larger the redox potential, the more oxidized the system is; conversely the smaller the redox potential, the more reduced the system is. At pH 5, the approximate pH of the TNX OD soils, the expected redox range is between 50 (reduced) and 650 mV (oxidized). In the metallic iron system, the pH rose to 10.2 and the oxidation-reduction potential (or redox) dropped to 110 mV (Table 12). The rather large metallic-iron reduction potential is appreciably larger than expected; values of ~ 200 are common. Faust and Osman (1981) reported similar reductions in soluble Hg concentrations in experimental systems when pH values increased and Eh values decreased. As described above, the elevated pH in the metallic iron system can be attributed to the water being reduced to hydrogen gas and hydroxide ions. The low pH of the pyrite addition can be attributed to the oxidation of pyrite, which is a well known acidifying process that is a widespread occurrence at coal mining sites.
Table 12. Effect of sequestering materials on aqueous Hg concentration.(a)
|
Treatment |
6-mg/L Total Organic Carbon |
>100-mg/L Total Organic Carbon |
|||||||||
|
Avg. pH |
Avg. Eh |
Avg. Hg (mg/L) |
Kd |
Avg. pH |
Avg. Eh |
Avg. Hg (mg/L) |
Kd |
||||
|
Blank Control |
5.8 |
409 |
0.000 |
-- |
5.0 |
290 |
0.001 |
-- |
|||
|
Hg Spike Control |
3.1 |
498 |
1.865 |
-- |
4.7 |
311 |
0.630 |
-- |
|||
|
Gypsum Addition |
3.3 |
510 |
1.582 |
17 ± 0 |
4.5 |
330 |
0.344 |
448 ± 56 |
|||
|
Pyrite Addition |
3.0 |
345 |
0.002 |
108838 ± 26457 |
4.3 |
361 |
0.009 |
20259 ± 413 |
|||
|
Metallic iron Addition |
10.2 |
100 |
0.029 |
6270 ± 719 |
5.8 |
110 |
0.127 |
1364 ± 117 |
|||
|
(a) 0.3 g solid: 30-mL SRS surface water; 1-wk equilibration period; 3 replicates; total mass of mixtures = 0.3 g evenly divided between sequestering agent. High organic matter concentration tests were conducted with leaf litter leachate solution; low organic matter concentration tests were conducted with SRS surface groundwater with 6 mg/L TOC. (b) Average ± standard deviation. |
|||||||||||
In the third experiment, six elements were added to the solution at concentrations of approximately 50 m g/L. After one week the concentrations of each tested element were significantly reduced by almost all the amendments (Table 13).
Table 13. Kd values (mL/g) of Co, Ba, Eu, Pb, and U for various potential sequestering agent materials.(a)
|
Treatment |
Co |
Ba |
Eu |
Pb |
U |
|
Hydroxylapatite |
7683 |
421 |
725426 |
138607 |
282448 |
|
North Carolina Apatite |
470 |
222 |
313157 |
214916 |
264829 |
|
Zeolite (Clinoptilolite) |
4114 |
6217 |
14473 |
1805864 |
7 |
|
Zeolite (Phillipsite) |
223 |
6222 |
819 |
24828 |
9 |
|
Fe oxide (waste by product) |
23037 |
3688 |
2561951 |
363449 |
8479 |
|
Metallic Fe |
259629 |
26076 |
1525058 |
18108 |
452210 |
|
(a) 0.3 g solid: 30-mL SRS surface water; 1-wk equilibration period; 3 replicates; total mass of mixtures = 0.3 g evenly divided between sequestering agent. (b) Ba and Eu are used as analogues for Ra and Ac geochemical behavior. |
|||||
Hydroxylapatite and North Carolina Apatite were the most effective at reducing Eu, Pb, and U concentrations in the solutions and Kd values in these treatments were very high (Table 13). Other researchers also reported the high effectiveness of apatite in remediation of Pb or U contaminated soils through a precipitation mechanism (Knox et al. 2000). Both zeolites were effective for most elements, however, not for uranium. Metallic iron and Fe oxide (Fe-rich TM, waste byproduct) removed the greatest mass of the targeted constituents from solution. The metallic iron could be attractive as a geomat material because it was effective in immobilizing Cr, Hg, Co, Ba, Eu, Pb and U; however, one problem with metallic iron is that it has a limited life span before it oxidizes, rusts, and looses its reductive capacity. Thus, if metallic iron was to be included in a geomat, then the material would have to be removed before the metallic iron completely rusted.
4.5 Geomat Efficiency Field Experiment
The first part of this project, setting up in the field, was completed. Results will become available as soon as next spring.
4.6 Linear Kinetic Reservoir Model
The linear kinetic reservoir model was not run because not all the analytical data is available. Modeling will be the last thing to be completed prior to writing up the next version of this report.
A modeling effort has been undertaken to estimate the timeframe that will be required to remediate the TNX Outfall Delta to acceptable risk levels and to focus future work on the phytoimmobilization concept on the biogeochemical parameters that are driving contaminant immobilization. For this effort, the linear kinetic reservoir approach of Lasaga (1980) is being implemented. In this method, the time-dependant cycling of contaminants in the TNX Outfall Delta is evaluated by a matrix algebra solution of a series of competing transfer reactions between contaminant reservoirs. The mass of contaminant, reaction pathway (i.e., the reservoirs to which contaminant can be transferred), and the rate that contaminants are transferred between reservoirs determine the distribution between each of the reservoirs.
The laboratory and field studies have been designed to provide model input data for this analysis. In our model conceptualization the reservoirs considered are the root-zone soil, bulk soil (below root zone), vegetation, and the reactive geomat. A general model schematic is shown in Figure 10 and the data collected in this study are designed to provide the mass of contaminant in each reservoir and the rate of transfer between the reservoirs as designated by the arrow. The matrix solution of Lasaga (1980) can then be used to calcuate the distribution of contaminants between each of the reservoirs as a fuction of time.
In preparation for the modeling, input values for the model have been assembled based on based available data (Table 14 and Table 15). These tables are continuously updated and will again be updated once the remainder of the analytical data becomes available.
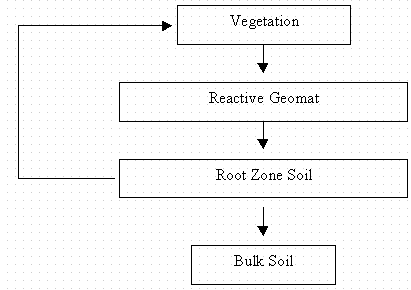
In assembling this data, a number of decisions had to be made. For the sequestering agent, it was decided that metallic iron would not be used, even though it had high Kd values. The reason for this decision was that if the metallic iron was used, it would have to be removed at a rather frequent basis because of its tendency to rust, thereby changing into a poorer sequestering agent. Consequently, the geomat that was selected consisted of a hydroxyapatite, clinoptilolite, and pyrite.
One concern is that instead of using the measured Kd values for Hg, Pb, Th, and U, perhaps solubility values should be used. The sequestering agents are removing these contaminants by forming the sparingly soluble phases of Hg-S, Pb-PO4, ThO2 or Th-PO4, and U-PO4. The implications of using a solubility versus a sorption construct is that the contaminant solution concentration will remain constant for as long as the solid source material exists. Once the solid phase controlling solubility is totally disolved, the contaminant concentration drops to zero. With the sorption, or Kd construct, the contaminant solution concentration varies directly to the concentration in the aqueous phase. The contaminant concentration in the Kd construct, slowly decreases, all the time maintaining a fixed ratio of contaminant concentration on the solid phase to the liquid phase.
The proposed input data also lists the highest soil concentration measured in the TNX OD. This "conservatism" may not be necessary; perhaps a better choice for soil concentration values would be to pick the contaminant concentration from a single site, such as the most contaminated site near coordinate A-5. No one site has the highest concentrations of all contaminants.
Table 14. Preliminary Input Values for the Linear Kinetic Reservoir Model of Phytoimmobilization (Additional input in next table).
|
Parameter |
Parameter |
Values |
Units |
Comments |
|
Biomass Flux |
Annual leaf litter flux |
2000 |
kg/ha/yr |
Based on litter collected in & beneath baskets; baskets put out in middle of fall. Control sites only. |
|
Annual fern biomass biomass flux |
610 |
kg/ha/yr |
Assumptions: 17 g/plant (actual is 10 to 25 g/plant); 1.5 ft2/plant, 50% cover |
|
|
Geomat Kd's |
Ac-Kd |
725426 |
mL/g |
Hydroxyapatite/Clinoptilolite/Sulfur no OM data, Used Eu as nonrad analog in experiments |
|
Co-Kd |
7683 |
mL/g |
Hydroxyapatite/Clinoptilolite/Sulfur no OM data. |
|
|
Cr-Kd |
15 |
mL/g |
Hydroxyapatite/Clinoptilolite/Sulfur. If we add Fe(0) the Geomat Kd greatly increases to 200 mL/g. |
|
|
Hg-Kd |
20259 |
mL/g |
Pyrite/Leaf Leachate data (Prague 2000); Acidifying nature of pyrite may adversely affect plant growth. |
|
|
Pb-Kd |
138607 |
mL/g |
Hydroxyapatite/Clinoptilolite/Sulfur no OM data |
|
|
Ra-Kd |
6217 |
mL/g |
Hydroxyapatite/Clinoptilolite/Sulfur no OM data, Used Ba as nonrad analog in experiments |
|
|
Th-Kd |
5700 |
mL/g |
Actually >5700 mL/g, Hydroxyapatite/Clino/Sulfur no OM data, detection limitation |
|
|
U-Kd |
282448 |
mL/g |
Hydroxyapatite/Clinoptilolite/Sulfur no OM data |
|
|
Soil Kds |
Ac-Kd |
255 |
mL/g |
Conservative Kd from desorption Kd of site specific sediment, WSRC-TR-99-00490 Table 16, Ce as analog |
|
Co-Kd |
255 |
mL/g |
Conservative Kd from desorption Kd of site specific sediment, WSRC-TR-99-00488 Table 16, Ni as analog |
|
|
Cr-Kd |
58 |
mL/g |
Conservative Kd from desorption Kd of site specific sediment, WSRC-TR-99-00494 Table 16 |
|
|
Hg-Kd |
4704 |
mL/g |
Conservative Kd from desorption Kd of site specific sediment, WSRC-TR-99-00494 Table 16 |
|
|
Pb-Kd |
11460 |
mL/g |
Conservative Kd from desorption Kd of site specific sediment, WSRC-TR-99-00494 Table 16 |
|
|
Ra-Kd |
336 |
mL/g |
Conservative Kd from desorption Kd of site specific sediment, WSRC-TR-99-00489 Table 16, Ba as analog |
|
|
Th-Kd |
115 |
mL/g |
Conservative Kd from desorption Kd of site specific sediment, WSRC-TR-99-00494 Table 16 |
|
|
U-Kd |
170 |
mL/g |
Conservative Kd from desorption Kd of site specific sediment, WSRC-TR-99-00494 Table 16 |
|
|
Soil Conc. |
Ac-228 |
101 |
pCi/g |
Conservative, Selected greatest Total Concentration (acid digest) from maps in WSRC-RP-4158, Rev. 0 |
|
Co-60 |
??? |
pCi/g |
||
|
Cr |
156 |
mg/kg |
Conservative, Selected greatest Total Concentration (acid digest) from maps in WSRC-RP-4158, Rev. 0 |
|
|
Hg |
30.8 |
mg/kg |
Conservative, Selected greatest Total Concentration (acid digest) from maps in WSRC-RP-4158, Rev. 0 |
|
|
Pb-212 |
97.1 |
pCi/g |
Conservative, Selected greatest Total Concentration (acid digest) from maps in WSRC-RP-4158, Rev. 0 |
|
|
Ra-228 |
106 |
pCi/g |
Conservative, Selected greatest Total Concentration (acid digest) from maps in WSRC-RP-4158, Rev. 0 |
|
|
Th-232 |
52.5 |
pCi/g |
Conservative, Selected greatest Total Concentration (acid digest) from maps in WSRC-RP-4158, Rev. 0 |
|
|
Th-232 |
694 |
mg/L |
Conservative, Selected greatest Total Conc. (acid digest) from 6 sed.; WSRC-TR-99-00488 Tables 14/15 |
|
|
U-238 |
559 |
mg/kg |
Conservative, Selected greatest Total Conc. (acid digest) from 6 sed.; WSRC-TR-99-00488 Tables 14/15 |
|
|
U-238 |
123 |
pCi/g |
Conservative, Selected greatest Total Concentration (acid digest) from maps in WSRC-RP-4158, Rev. 0 |
Table 15. Preliminary Input Values for the Linear Kinetic Reservoir Model of Phytoimmobilization (Additional input in previous table).
|
Parameter Category |
Parameter |
Values |
Units |
Comments |
|
Leaf/soil |
Ac-Leaf Litter CR |
na |
ppm/ppm |
Not available yet |
|
Co-Leaf Litter CR |
46.22 |
ppm/ppm |
Plant material and sediment from A-5 |
|
|
Cr-Leaf Litter CR |
0.24 |
ppm/ppm |
Plant material and sediment from A-5 |
|
|
Hg-Leaf Litter CR |
2.93E-05 |
ppm/ppm |
Plant material and sediment from A-5 |
|
|
Pb-Leaf Litter CR |
0.16 |
ppm/ppm |
Plant material and sediment from A-5 |
|
|
Ra-Leaf Litter CR |
21.16 |
ppm/ppm |
Plant material and sediment from A-5, Ba analog |
|
|
Th-Leaf Litter CR |
0.01 |
ppm/ppm |
Plant material and sediment from A-5 |
|
|
U-238 Leaf Litter CR |
0.15 |
ppm/ppm |
Plant material and sediment from A-5 |
|
|
Ac-Fern CR |
na |
ppm/ppm |
Not available yet |
|
|
Co-Fern CR |
6.542842 |
ppm/ppm |
Plant material and sediment from A-5 |
|
|
Cr-Fern CR |
1.056054 |
ppm/ppm |
Plant material and sediment from A-5 |
|
|
Hg-Fern CR |
1.169916 |
ppm/ppm |
Plant material and sediment from A-5 |
|
|
Pb-Fern CR |
0.403977 |
ppm/ppm |
Plant material and sediment from A-5 |
|
|
Ba-Fern CR |
29.75858 |
ppm/ppm |
Plant material and sediment from A-5; Ba analog |
|
|
Th-Fern CR |
1.069652 |
ppm/ppm |
Plant material and sediment from A-5 |
|
|
U-238 Fern CR |
1.102824 |
ppm/ppm |
Plant material and sediment from A-5 |
5.0 Conclusions
The manner in which phytoimmobilization should be deployed at the site is to use both the natural leaf litter, which generates a lot of biomass with moderate levels of contaminants, and plant ferns to phytoextract the contaminants near the soil surface, where the contaminants are most concentrated. The geomat should be made up of a combination of hydroxyapatite, clinoptilolite, and a sulfide source. The geomat should be fabricated by placing the sequestering agent in between two pieces of geotextile material, and then placed in pieces around the existing trees.
Leaf litter at the site was found to contain measurable concentrations of the constituents of concern (COCs; actinium, cobalt, chromium, mercury, lead, radium, thorium and uranium). Equally important, the leaf litter at the site was found to have a large annual biomass flux, 2000 kg/ha/yr. As part of a preliminary survey of the indigenous plants, it was discovered that a fern, Woodwardia areolata, contained exceptionally high contaminant concentrations. The species was particularly effective at accumulating cobalt and chromium. It also removed high concentrations of lead, uranium and thorium, and low concentrations of mercury. A greenhouse study is underway that will further quantify contaminant accumulations by the ferns.
In separate laboratory studies, sequestering agents were evaluated. Pyrite, a sulfide mineral, was found to have a distribution coefficient (Kd value) of 20,000 mL/g for mercury. Hydroxyapatite, a phosphate source, was able to remove large amounts of cobalt (Kd = 7700 mL/g), europium (an analogue for actinium; Kd = 720,000), lead (Kd = 138,000 mL/g), and uranium (Kd = 282,000 mL/g). Clinoptilolite, a zeolite cation exchange mineral, effectively removed barium (an analogue for radium; Kd = 6200 mL/g). A field demonstration of the various sequestering agent has been set up at the TNX OD.
This proposed approach to remediating the ecologically sensitive wetland has a number of attributes. First, this approach uses existing natural geocycling processes and simply interrupts these processes by accumulating the contaminants in the geomat. Secondly, this approach should have minimal impact on the ecologically sensitive wetland at the TNX OD site; the geomat may be removed or left in place, depending on the risk. Finally, the technology should greatly reduce the cost of waste disposal by creating a concentrated waste in the sequestering agent.
6.0 Acknowledgment
The information contained in this report was developed during the course of work under Contract No. DE-AC09-96SR18500 with the U.S. Department of Energy. Dr. Rebecca Sharitz and Mr. Bruce Allen (Savannah River Ecology Laboratory, Aiken SC) contributed greatly to this research but were unable to review the document before it was issued. They will be co-authors on the next version of this document.
7.0 References
Bodek, W.J. Lymn, W.F. Reehel, D.H. Rosenblott, B.I. Walton, R.A. Conway, Environmental Inorganic Chemistry: Properties, Processes, and Estimation Methods, New York, Pergamon Press, (1988).
Cantrell, K. J., and D. I. Kaplan. 1998. "Sorptive Barriers for Groundwater Remediation." pp. 4663 - 4677. In Robert A. Meyers (ed.), Encyclopedia of Environmental Analysis and Remediation, John Wiley & Sons, Inc., New York.
Cantrell, K. J., D.I. Kaplan, D.I., T. W. Wietsma, J. Hazardous Materials. 42, 201-212, (1995).
Faust, S. D. In: Chemistry of Natural Waters, Ann Arbor Science Publishers, Inc., (1981).
Kaplan, D. I. Sampling and Analyses Plan for the Phytostabilization Study at the TNX Outfall Delta, Lower Discharge Gully and Swamp Operable Unit. WSRC-RP-99-01078. Westinghouse Savannah River Company, Aiken SC (1999).
Kaplan, D. I., S. V. Mattigod, K. E. Parker, and G. Iversen. I-129 Test and Research to Support Disposal Decisions. WSRC-TR-2000-00283, Rev. 0, Westinghouse Savannah River Company, Aiken, SC (2000).
Kaplan, D. I., and S. M. Serkiz. In-situ Kd Values and Geochemical Behavior for Inorganic and Organic Constituents of Concern at the TNX Outfall Delta. WSRC-TR-99-00488. Westinghouse Savannah River Company, Aiken, SC (1999).
Knox, A. S, J.C. Seaman, M.J. Mench, J. Vangronsveld, 2000, Remediation of Metal- and Radionuclide-Contaminated Soils by In Situ Stabilization In: Environmental Restoration of Metals Contaminated Soils, Ed. IK Iskandar, CRC Press, Boca Raton, FL., (2000).
Lasaga, A. C. 1980. "The Kinetic Treatment of Geochemical Cycles." Geochimica et Cosmochimica Acta. 44, p 815 (1980).
WSRC. RFI/RI with BRA for the TNX Outfall Delta, Lower Discharge Gully and Swamp Operable Unit. WSRC-RP-98-4158, Rev. 0. Westinghouse Savannah River Company, Aiken, SC. (1999).