Strontium
The principal mechanism for production of strontium was neutron-induced fission in the reactors. When a reactor was operating, neutron-induced fission reactions occurred in the 235U fuel of the reactor core. Fission reactions formed a variety of fission products, of which strontium was one of the most important.(br>
Strontium was not observed in atmospheric releases from the reactors. Most strontium released to the atmosphere came from the separation process in F Area and H Area. In contrast, most of the strontium released to streams came from basin purges in the reactor areas. Releases of unidentified beta-gamma occurred primarily from A Area and were assumed to be 90Sr for dose calculations.
Zirconium-Niobium-95, 106Ru, 144Ce
The principal mechanism for production of 95Zr, 95Nb,
106Ru, and 144Ce was neutron-induced fission in the
reactors. When a reactor was operating, neutron-induced fission reactions
occurred in the 235U fuel of the reactor core. Fission reactions
formed a variety of fission products, which included those listed above.
Fission products rarely were seen in atmospheric releases from the reactors. Most fission products released to the atmosphere resulted from the separation process in F Area and H Area. In contrast, most of the fission products released to streams came from basin purges in the reactor areas.
Technetium
There were virtually no measurements of 99Tc releases. Release quantities have been conservatively estimated.
Iodine-129 and Iodine-131
The principal mechanism for production of 129I and 131I
was neutron-induced fission in the reactors. When a reactor was operating,
neutron-induced fission reactions occurred in the 235U fuel of the
reactor core. Fission reactions formed a variety of fission products, which
included several isotopes of iodine. The two largest contributors to
environmental dose were 129I and 131I.
Iodine was released to the atmosphere when the fuel and target elements were chemically dissolved in F Area and H Area. The quantity of 131I released depended on the cooling time between reactor shutdown and dissolution of the elements. Cooling times were much shorter during the 1950s, when there was a greater sense of production urgency.
Cesium-137
The principal mechanism for production of 137Cs was neutron-induced
fission in the reactors. When a reactor was operating, neutron-induced fission
reactions occurred in the 235U fuel of the reactor core. Fission
reactions formed a variety of fission products, which included isotopes of
cesium. Additional 137Cs was formed in the reactor as a result of
neutron activation of stable cesium generated by neutron fission.
There were no recorded atmospheric 137Cs releases from the reactors.
Most of the atmospheric 137Cs released from the separation areas was
the result of two incidents. The first occurred in 1955 during startup,
primarily as a result of leakage around the sand filter bypass plug. The second
occurred in 1987, when an evaporator steam flange failed in the waste
management facility.
Most of the liquid 137Cs releases were from the reactors as a result of leaking fuel elements in the 1950s and 1960s. The fuel elements were stored in disassembly basins, and 137Cs was released to site streams when basin water was purged to maintain clarity and remove heat. Approximately two-thirds remain in the stream beds, flood plains, ponds, and swamps on or near SRS.
Uranium
Uranium releases generally have been associated with the fabrication of reactor fuel and target elements (M Area) and with the chemical processing of spent target and fuel material (F Area and H Area).
Plutonium
Plutonium at SRS was formed during the irradiation of nuclear fuel and targets
during operation of the site's five production reactors.
Atmospheric plutonium releases occurred primarily in F Area and H Area and were largest during startup of the canyon facilities in 1955. Unidentified alpha releases from the reactors and other facilities were assumed to be plutonium. Approximately 70 % of atmospheric plutonium releases occurred in 1955.
Curium-244
Beginning in 1963, transplutonium isotopes were prepared by placing
239Pu targets in high-flux charges in SRS reactors. After the
targets were dissolved and processed in a separation facility, they were
delivered to SRTC for further processing. The work involved gram quantities of
curium and americium, microgram quantities of californium and berkelium, and
nanogram quantities of einsteinium. By 1968, approximately 5 kg of
244Cm had been recovered (Moyer 1968). The 244Cm was
used in an experimental program as a heat source for isotopic electrical power
generators (Stoddard 1964).
Atmospheric 244Cm releases were reported for F Area and H Area, but the majority of released material came from A Area during the years when research was conducted on the use of 244Cm as a heat source for electricity generation.
Surface Water Transport
SRS is drained by five streams that flow into the Savannah River. Except for 137Cs, it was conservatively assumed that the radionuclides released to site streams were not adsorbed or deposited in the streambeds or in the Savannah River. Site specific studies have shown that only 35% of 137Cs released to streams was transported to the Savannah River (Carlton 1994). A description of the site streams has been published previously (Carlton 1994).
Dose to Humans
SRS offsite doses were calculated with the transport and dose models developed for the commercial nuclear industry (USNRC 1977a; USNRC 1977b). The models are implemented at SRS in the following computer programs:
Atmospheric Releases
- MAXIGASP-calculates maximum and average doses to offsite individuals.
- POPGASP-calculates offsite population collective dose.
Liquid Releases
- LADTAP XL-calculates both maximum and average doses to offsite individuals and collective dose to offsite populations.
MAXIGASP and POPGASP are SRS-modified versions of the Nuclear Regulatory
Commission (NRC) programs XOQDOQ (Sagendorf et al. 1982) and GASPAR (Eckerman
et al. 1980). The modifications were made to meet the requirements for input
of physical and biological data that are specific to SRS. The basic
calculations in the XOQDOQ and GASPAR programs have not been modified. LADTAP
XL is a spreadsheet version of LADTAP II (Simpson and McGill 1980).
In 1988, the U. S. Department of Energy (DOE) issued dose conversion factors to ensure that doses are calculated in a consistent manner at all DOE facilities (U.S. DOE 1988). The factors are based on ICRP recommendations (ICRP 1979) and were used in conjunction with the models described to calculate all doses.
Modeling atmospheric dispersion of radioactive releases
The routine atmospheric transport of radioactive materials from SRS is
evaluated on the basis of meteorological conditions measured continuously at
nine onsite towers. A database containing the 60-min average values for the
period 1987-1991 is accessed by the dispersion codes to estimate downwind
concentrations of released radionuclides. Offsite doses have been calculated
assuming two release points. Doses were calculated for A-Area releases (near
the edge of the site) using A-Area meteorology. Doses were calculated for the
remainder by assuming the releases occurred at the geographic center of the
site and using meteorology measured by the H-Area tower.
The dispersion of atmospheric releases from SRS was modeled using XOQDOQ, which
estimates concentrations in the plume as a function of downwind distance and
compass sector. The model takes into account depletion due to dry deposition
and radioactive decay.
The doses estimated by GASPAR are reported on a pathway-specific basis as follows:
- Plume-external dose from radioactive materials suspended in the
atmosphere
- Ground-external dose from radioactive materials deposited on the
ground
- Inhalation-internal dose from inhalation of radioactive materials
present in the plume
- Vegetation-internal dose from consumption of contaminated crops
- Milk-internal dose from consumption of milk produced in a contaminated
area
- Meat-internal dose from consumption of meat produced in a contaminated
area.
Additional information on the details of the modeling are available (Carlton et al. 1994).
Modeling doses from liquid releases
The consequences of liquid releases from SRS were modeled using LADTAP XL. Pathway-specific doses are grouped into the following four categories:
- Potable drinking water-internal dose from consuming drinking water of
Savannah River origin
- Sport fish and commercial fish-internal dose from consuming fish of
Savannah River origin
- Salt water invertebrates-internal dose from consuming shell fish from
the Savannah River estuary
- Recreation-external dose from recreation activities in and along the
Savannah River (boating, swimming, and shoreline activities).
LADTAP XL estimates individual and population doses at specific downriver
locations. The only removal mechanism included in the transport model, as it
is used at SRS, is radioactive decay. No credit is taken for adsorption on
stream sediments or removal by the water treatment process at the downriver
water treatment plants.
The major assumption inherent in the application of LADTAP XL to SRS releases is that liquid discharges undergo complete mixing in the Savannah River before reaching potentially exposed populations. This assumption is supported by repeated measurements indicating that complete mixing occurs in the 16 river km between Lower Three Runs and the Highway 301 sampling station (Cummins et al. 1991a). The nearest water treatment plant is 140 river km downstream from SRS.
LADTAP XL generates maximum individual and population doses for all of the exposure pathways identified above. SRS calculations are performed with site-specific information. Radioisotope concentration in the Savannah River is decreased by the inflow of streams downriver from SRS. Additional dilution occurs at the Beaufort-Jasper water treatment plant due to the inflow of surface water and at the Port Wentworth water treatment plant due to the close proximity of Abercorn Creek to the intake. Since SRS-released tritium is readily measured in the Savannah River, and in the processed water of each system, a derived river flow rate based on simple dilution was calculated. This allows more accurate estimates of strontium concentrations at these treatment plants.
Doses from atmospheric releases
As shown in Table 2, the effective dose equivalent theoretically received by
the maximally exposed adult individual was 770 µSv. The radionuclide
contributing the highest portion of this dose was 131I, followed by
3H, Pu (includes 238Pu, 239Pu, and gross
alpha), and 41Ar.
A person living in the SRS area would have received an effective dose of
approximately 1.2 x 105 µSv from exposure to natural sources of
radioactivity and an additional 2.6 x 104 µSv from medical
practices and various consumer products during the 43 y period (Cummins et al.
1991b).
The population doses in Table 3 were based on 1980 census data (555,100 people within 80 km) and current meteorological and dose factor data. It was assumed that this population lived in the SRS vicinity (within 80 km) throughout the period of site operation. The total collective effective dose from atmospheric releases through 1996 was 32 person-Sv.
Doses from liquid releases
Dose equivalents were calculated for the maximally exposed individual living
just downstream from SRS who subsisted on a diet of untreated Savannah River
water and fish of Savannah River origin (Table 2). The total dose was 1.4 x
103 µSv with 137Cs and 32P contributing
75% the dose.
Since an individual's dose from non-SRS sources of radiation for that same time
period was almost 1.5 x 105 µSv, it may be concluded that the
contribution to downstream individual doses by SRS releases is insignificant.
The total population dose for liquid releases is the sum of the dose from the
water treatment plants pathway (1.9 person-Sv, 65,000 people) plus the dose due
to other liquid pathways such as fish and recreation (15 person-Sv, 555,100
people). The collective dose equivalent is 17 person-Sv distributed among
620,100 people.
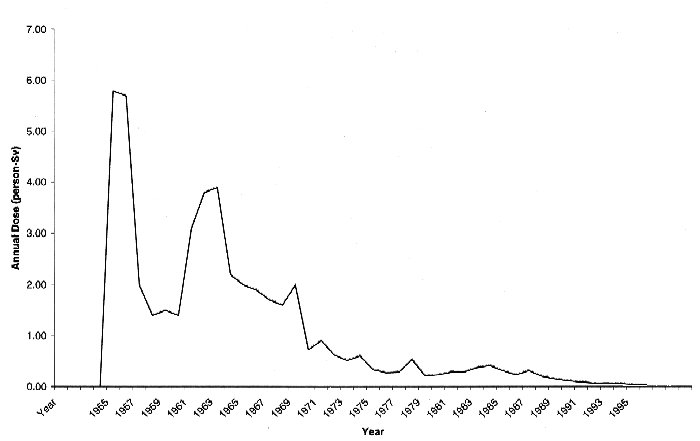
The total population dose from both atmospheric and liquid releases is shown by year in Table 4 and is graphically depicted in Fig. 1. The highest years were 1955 and 1956 when atmospheric 131I and Pu releases occurred. Population dose has declined over the years, decreasing by a factor of 10 by 1973 and by a factor of 100 by 1995.
References
Albenesius, E.L., 1959, "Tritium as a Product of Fission", Phy. Rev. Let.
3(6):274.
Angerman, C.L., 1973, "Savannah River Laboratory Cobalt-60 Power and Heat
Sources, Final Quarterly Progress Report," DP-1338, E.I. duPont de Nemours
& Co., Aiken, SC.
Arnett, M.W., L.K. Karapatakis, A.R. Mamatey, and J.L. Todd, 1992, "Savannah
River Site Environmental Report for 1991," WSRC-TR-92-186, Westinghouse
Savannah River Company, Aiken, SC.
Arnett, M.W., L.K. Karapatakis, and A.R. Mamatey, 1993, "Savannah River Site
Environmental Report for 1992," WSRC-TR-93-075, Westinghouse Savannah River
Company, Aiken, SC.
Arnett, M.W., L.K. Karapatakis, and A.R. Mamatey, 1994, "Savannah River Site
Environmental Report for 1993," WSRC-TR-94-075, Westinghouse Savannah River
Company, Aiken, SC.
Arnett, M.W., A.R. Mamatey, and D. Spitzer, 1995, "Savannah River Site
Environmental Report for 1994," WSRC-TR-95-075, Westinghouse Savannah River
Company, Aiken, SC.
Arnett, M.W., and A.R. Mamatey, 1996, "Savannah River Site Environmental Report
for 1995," WSRC-TR-96-0075, Westinghouse Savannah River Company, Aiken, SC.
Arnett, M.W., and A.R. Mamatey, 1997, "Savannah River Site Environmental Report
for 1996," WSRC-TR-97-0171, Westinghouse Savannah River Company, Aiken, SC.
vAshley, C., 1966, "Environmental Monitoring at the Savannah River Plant, Annual
Report - 1966," DPST-67-302, E.I. duPont de Nemours & Co., Aiken, SC.
Carlton, W. H.; Evans, A. G.; Geary, L. A.; Murphy, C. E., Jr.; Strom, R. N.
Assessment of strontium in the Savannah River Site environment. Aiken, SC:
Westinghouse Savannah River Company; Report No. WSRC-RP-92-984, 1992.
Carlton, W. H.; Murphy, C. E., Jr.; Evans, A. G. Radiocesium in the Savannah River Site environment. Health Phys. 67:233-244; 1994.
Carlton, W. H.; Murphy, C. E., Jr.; Evans, A. G. Plutonium in the Savannah River Site environment. Health Phys. 71:290-299; 1996.
Carlton, W. H., Murphy, C. E., Jr., G. T. Jannik, Simpkins, A. A.
Radiostrontium in the Savannah River Site Environment. Health Phys. (In
Press), 1999.
Cummins, C.L., D.K. Martin, and J.L. Todd, 1991a, "Savannah River Site
Environmental Report, 1990," WSRC-IM-91-28, Westinghouse Savannah River
Company, Aiken, SC.
Cummins, C.L., C.S. Hetrick, and D.K. Martin, 1991b, "Radioactive Releases at
the Savannah River Site 1954-1989," WSRC-RP-91-684, Westinghouse Savannah River
Company, Aiken, SC.
Eckerman, K.F.; Congel, F.J.; Roecklein, A.K.; Pasciak, W.J. User's guide to
GASPAR code. Springfield, VA: National Technical Information Service; Report
No. NUREG-0597; 1980.
Fox, L.W., 1975, "Radioactive Releases in Excess of Annual Guides,"
SRT-ETS-960031, E.I. duPont de Nemours & Co., Aiken, SC.
Hayes, D.W., and K.W. MacMurdo, 1977, "Carbon-14 Production by the Nuclear
Industry," Health Physics, 32: 215-219.
International Commission on Radiological Protection. Limits for intake of
radionuclides by workers. Oxford: Pergamon Press; ICRP Publication 30, Part 1;
1979.
Longtin, F.B., 1966, "Moderator Silicate Control of 32P Release,"
DPSOX-6546, E.I. duPont de Nemours & Co., Aiken, SC.
Longtin, F.B., 1972, "100-Area Release Guides for 95Zr-Nb and
51Cr," SRT-ETS-960029, E.I. duPont de Nemours & Co., Aiken,
SC.
Moyer, R.A., 1968, "Savannah River Experience with Transplutonium Elements,"
Health Physics 15: 133-138.
Sagendorf, J.F.; Goll, J.T.; Sandusky, W.F. XOQDOQ: Computer program for the
meteorological evaluation of routine effluent releases at nuclear power
stations. Springfield, VA: National Technical Information Service; Report No.
NUREG/CR-2919; 1982.
Simpson, D.B.; McGill, B.L. Users Manual for LADTAP II-A computer program for
calculating radiation exposure to man from routine releases of nuclear reactor
effluents. Springfield, VA: National Technical Information Service; Report No.
NUREG/CR-1276; 1980.
D.H. Stoddard, 1964, "Radiation Properties of 244Cm Produced for
Isotopic Power Generators," DP-939, E.I. du Pont de Nemours & Company,
Savannah River Laboratory, Aiken, SC.
U.S. Department of Energy. Internal dose conversion factors for calculation of
dose to the public. Springfield, VA: National Technical Information Service;
Report No. DOE/EH-0071; 1988.
Zecha, D.J., 1987, "60Co Fueled Thermoelectric Generator Burial," DPSOX-10051, E.I. duPont de Nemours & Co., Aiken, SC.
Radionuclide |
Atmospheric Releases (GBq) |
Liquid Releases (GBq) |
3H |
9.6 x 108 |
5.9 x 107 |
14C |
1.1 x 105 |
|
32P |
1.3 x 103 | |
41Ar |
2.4 x 108 |
|
51Cr |
1.7 x 105 | |
60Co |
3.4 x 100 |
2.4 x 103 |
65Zn |
5.2 x 103 | |
Sr |
1.1 x 102 |
5.6 x 103 |
95Zr,Nb |
4.8 x 103 | |
99Tc |
4.1 x 102 |
|
106Ru |
5.2 x 103 |
2.2 x 103 |
129I |
2.1 x 102 |
|
131I |
9.3 x 104 |
1.1 x 104 |
137Cs |
1.3 x 102 |
7.8 x 103 |
144Ce |
1.3 x 104 | |
U* | 3.2 x 101 |
9.3 x 102 |
Pu** | 1.4 x 102 |
2.7 x 101 |
244Cm |
3.3 x 100 |
1.4 x 101 |
*Natural Uranium analyzed as 238U
**Includes 238Pu, 239Pu, and gross alpha
Radionuclide |
Atmospheric
Dose |
Percent
of |
Liquid
Dose |
Percent
of |
3H |
1.8 x 102 |
23.3 |
7.9 x 101 |
5.5 |
14C |
9.8 x 100 |
1.3 |
||
32P |
4.6 x 102 |
32.1 | ||
41Ar |
7.8 x 101 |
10.1 |
||
51Cr |
2.2 x 100 |
0.2 | ||
60Co |
7.6 x 100 |
1.0 |
4.4 x 100 |
0.3 |
65Zn |
7.3 x 101 |
5.1 | ||
Sr |
7.2 x 101 |
9.3 |
2.5 x 101 |
1.7 |
95Zr,Nb |
1.5 x 102 |
10.5 | ||
99Tc |
1.0 x 100 |
0.1 |
||
106Ru |
4.4 x 101 |
5.7 |
1.1 x 100 |
0.1 |
129I |
3.9 x 101 |
5.1 |
||
131I |
2.1 x 102 |
27.2 |
1.5 x 101 |
1.0 |
137Cs |
4.7 x 100 |
0.6 |
6.1 x 102 |
42.6 |
144Ce |
4.7 x 100 |
0.3 | ||
U* | 4.2 x 100 |
0.5 |
4.7 x 100 |
0.3 |
Pu** | 1.2 x 102 |
15.5 |
2.8 x 100 |
0.2 |
244Cm |
1.5 x 100 |
0.2 |
1.1 x 100 |
0.1 |
Total |
7.7 x 102 |
1.4 x 103 |
*Natural Uranium analyzed as 238U
**Includes 238Pu, 239Pu, and gross alpha
Radionuclide |
Atmospheric |
Percent
of |
Liquid
Dose |
Percent
of |
Total
Dose |
Percent
of |
3H |
1.1 X 101 |
34.6 |
1.3 X 100 |
7.9 |
1.2 X 101 |
25.1 |
14C |
3.0 X 10-1 |
0.9 |
3.0 X 10-1 |
0.6 | ||
32P |
1.1 X 100 |
6.7 |
1.1 X 100 |
2.3 | ||
41Ar |
1.9 X 100 |
6.0 |
1.9 X 100 |
4.0 | ||
51Cr |
9.6 X 10-2 |
0.6 |
9.6 X 10-2 |
0.2 | ||
60Co |
6.5 X 10-3 |
0 |
2.8 X 10-1 |
1.7 |
2.9 X 10-1 |
0.6 |
65Zn |
1.1 X 101 |
66.0 |
1.1 X 101 |
23.0 | ||
Sr |
6.3 X 10-2 |
0.2 |
2.4 X 10-1 |
1.5 |
3.0 X 10-1 |
0.6 |
95Zr,Nb |
2.5 X 10-1 |
1.5 |
2.5 X 10-1 |
0.5 | ||
99Tc |
6.5 X 10-2 |
0.2 |
2.4 X 10-3 |
0 |
6.7 X 10-2 |
0.1 |
106Ru |
1.2 X 100 |
3.7 |
1.4 X 10-1 |
0.9 |
1.3 X 100 |
2.7 |
129I |
1.0 X 100 |
3.2 |
1.0 X 100 |
2.1 | ||
131I |
8.3 X 100 |
26.2 |
1.1 X 10-1 |
0.6 |
8.4 X 100 |
17.5 |
137Cs |
3.4 X 10-1 |
1.1 |
1.3 X 100 |
7.9 |
1.6 X 100 |
3.3 |
144Ce |
4.7 X 10-1 |
2.8 |
4.7 X 10-1 |
1.0 | ||
U* | 3.2 X 10-1 |
1.0 |
8.2 X 10-2 |
0.5 |
4.0 X 10-1 |
0.8 |
Pu** | 7.1 X 100 |
22.4 |
1.2 X 10-1 |
0.7 |
7.2 X 100 |
15.0 |
244Cm |
8.9 X 10-2 |
0.3 |
1.2 X 10-1 |
0.7 |
2.1 X 10-1 |
0.4 |
Total |
3.2 X 101 |
1.7 X 101 |
4.8 X 101 |
*Natural Uranium analyzed as 238U
**Includes 238Pu, 239Pu, and gross alpha
|
||||||
Year |
Dose
from |
Beaufort- |
Port |
80- |
Total |
Total |
1954 |
1.5 X 10-2 |
6.1 X 10-5 |
5.6 X 10-4 |
6.3 X 10-4 |
1.6 X 10-2 | |
1955 |
5.8 X 100 |
5.3 X 10-3 |
1.4 X 10-2 |
1.9 X 10-2 |
5.8 X 100 | |
1956 |
5.7 X 100 |
9.4 X 10-3 |
2.2 X 10-2 |
3.2 X 10-2 |
5.7 X 100 | |
1957 |
1.8 X 100 |
3.4 X 10-2 |
1.7 X 10-1 |
2.0 X 10-1 |
2.0 X 100 | |
1958 |
1.4 X 100 |
8.1 X 10-3 |
1.5 X 10-2 |
2.3 X 10-2 |
1.4 X 100 | |
1959 |
1.4 X 100 |
1.6 X 10-2 |
4.0 X 10-2 |
5.7 X 10-2 |
1.5 X 100 | |
1960 |
8.6 X 10-1 |
3.5 X 10-2 |
4.7 X 10-1 |
5.0 X 10-1 |
1.4 X 100 | |
1961 |
1.2 X 100 |
3.3 X 10-2 |
1.9 X 100 |
1.9 X 100 |
3.1 X 100 | |
1962 |
8.1 X 10-1 |
4.4 X 10-2 |
2.9 X 100 |
3.0 X 100 |
3.8 X 100 | |
1963 |
7.9 X 10-1 |
5.6 X 10-2 |
3.1 X 100 |
3.2 X 100 |
3.9 X 100 | |
1964 |
9.9 X 10-1 |
2.8 X 10-2 |
1.2 X 100 |
1.2 X 100 |
2.2 X 100 | |
1965 |
7.1 X 10-1 |
7.3 X 10-2 |
3.8 X 10-2 |
1.2 X 100 |
1.3 X 100 |
2.0 X 100 |
1966 |
6.5 X 10-1 |
9.8 X 10-2 |
3.9 X 10-2 |
1.1 X 100 |
1.2 X 100 |
1.9 X 100 |
1967 |
5.9 X 10-1 |
1.0 X 10-1 |
5.0 X 10-2 |
1.0 X 100 |
1.2 X 100 |
1.7 X 100 |
1968 |
7.4 X 10-1 |
9.5 X 10-2 |
4.0 X 10-2 |
7.1 X 10-1 |
8.5 X 10-1 |
1.6 X 100 |
1969 |
1.7 X 100 |
5.4 X 10-2 |
2.6 X 10-2 |
2.3 X 10-1 |
3.1 X 10-1 |
2.0 X 100 |
1970 |
5.1 X 10-1 |
2.8 X 10-2 |
2.2 X 10-2 |
1.7 X 10-1 |
2.2 X 10-1 |
7.3 X 10-1 |
1971 |
5.6 X 10-1 |
2.0 X 10-2 |
1.9 X 10-2 |
3.1 X 10-1 |
3.5 X 10-1 |
9.1 X 10-1 |
1972 |
5.6 X 10-1 |
2.8 X 10-2 |
1.6 X 10-2 |
2.9 X 10-2 |
7.3 X 10-2 |
6.3 X 10-1 |
1973 |
4.5 X 10-1 |
4.5 X 10-2 |
1.9 X 10-2 |
1.0 X 10-2 |
7.3 X 10-2 |
5.2 X 10-1 |
1974 |
5.3 X 10-1 |
3.7 X 10-2 |
2.0 X 10-2 |
1.8 X 10-2 |
7.6 X 10-2 |
6.1 X 10-1 |
1975 |
3.0 X 10-1 |
2.5 X 10-2 |
1.2 X 10-2 |
3.7 X 10-3 |
4.2 X 10-2 |
3.4 X 10-1 |
1976 |
2.4 X 10-1 |
2.4 X 10-2 |
1.5 X 10-2 |
2.5 X 10-3 |
4.1 X 10-2 |
2.8 X 10-1 |
1977 |
2.4 X 10-1 |
3.1 X 10-2 |
1.2 X 10-2 |
3.5 X 10-3 |
4.6 X 10-2 |
2.9 X 10-1 |
1978 |
5.0 X 10-1 |
2.1 X 10-2 |
1.4 X 10-2 |
1.8 X 10-3 |
3.6 X 10-2 |
5.4 X 10-1 |
1979 |
2.0 X 10-1 |
1.2 X 10-2 |
7.4 X 10-3 |
3.3 X 10-3 |
2.3 X 10-2 |
2.2 X 10-1 |
1980 |
2.2 X 10-1 |
1.2 X 10-2 |
6.2 X 10-3 |
1.3 X 10-3 |
1.9 X 10-2 |
2.4 X 10-1 |
1981 |
2.5 X 10-1 |
2.9 X 10-2 |
1.2 X 10-2 |
3.1 X 10-3 |
4.5 X 10-2 |
2.9 X 10-1 |
1982 |
2.5 X 10-1 |
2.3 X 10-2 |
1.3 X 10-2 |
2.7 X 10-3 |
3.9 X 10-2 |
2.9 X 10-1 |
1983 |
3.3 X 10-1 |
3.6 X 10-2 |
9.3 X 10-3 |
1.3 X 10-3 |
4.7 X 10-2 |
3.8 X 10-1 |
1984 |
3.9 X 10-1 |
1.8 X 10-2 |
8.1 X 10-3 |
3.6 X 10-3 |
3.0 X 10-2 |
4.2 X 10-1 |
1985 |
2.7 X 10-1 |
3.7 X 10-2 |
1.2 X 10-2 |
2.0 X 10-3 |
5.0 X 10-2 |
3.2 X 10-1 |
1986 |
1.8 X 10-1 |
4.6 X 10-2 |
1.5 X 10-2 |
2.7 X 10-3 |
6.3 X 10-2 |
2.4 X 10-1 |
1987 |
2.8 X 10-1 |
2.4 X 10-2 |
7.7 X 10-3 |
3.6 X 10-3 |
3.6 X 10-2 |
3.2 X 10-1 |
1988 |
1.6 X 10-1 |
3.6 X 10-2 |
1.1 X 10-2 |
4.1 X 10-3 |
5.1 X 10-2 |
2.1 X 10-1 |
1989 |
1.2 X 10-1 |
2.9 X 10-2 |
8.5 X 10-3 |
3.0 X 10-3 |
4.1 X 10-2 |
1.6 X 10-1 |
1990 |
9.2 X 10-2 |
1.5 X 10-2 |
5.6 X 10-3 |
2.6 X 10-3 |
2.3 X 10-2 |
1.2 X 10-1 |
1991 |
6.8 X 10-2 |
2.0 X 10-2 |
7.3 X 10-3 |
2.5 X 10-3 |
3.0 X 10-2 |
9.8 X 10-2 |
1992 |
5.0 X 10-2 |
1.8 X 10-2 |
6.6 X 10-3 |
2.2 X 10-3 |
2.6 X 10-2 |
7.7 X 10-2 |
1993 |
6.8 X 10-2 |
9.6 X 10-3 |
3.9 X 10-3 |
1.4 X 10-3 |
1.5 X 10-2 |
8.3 X 10-2 |
1994 |
5.6 X 10-2 |
1.1 X 10-2 |
4.1 X 10-3 |
2.7 X 10-3 |
1.8 X 10-2 |
7.4 X 10-2 |
1995 |
3.1 X 10-2 |
1.1 X 10-2 |
3.9 X 10-3 |
2.7 X 10-3 |
1.8 X 10-2 |
4.9 X 10-2 |
1996 |
2.6 X 10-2 |
1.4 X 10-2 |
4.6 X 10-3 |
4.0 X 10-3 |
2.2 X 10-2 |
4.9 X 10-2 |
Total |
3.2 X 10+1 |
1.1 X 100 |
7.5 X 10-1 |
1.5 X 10+1 |
1.7 X 10+1 |
4.8 X 10+1 |