WSRC-MS-99-00657
Development of the Am/Cm Batch Vitrification Process
D. K. Peeler, J. E. Marra, and I. A. Reamer
Westinghouse Savannah River Company
Aiken, SC 29808
J. D. Vienna and H. Li
Pacific Northwest National Laboratory
Hanford, Washington
This document was prepared in conjunction with work accomplished under Contract No. DE-AC09-96SR18500 with the U. S. Department of Energy.
DISCLAIMER
This report was prepared as an account of work sponsored by an agency
of the United States Government. Neither the United States Government nor any
agency thereof, nor any of their employees, makes any warranty, express or
implied, or assumes any legal liability or responsibility for the accuracy,
completeness, or usefulness of any information, apparatus, product or process
disclosed, or represents that its use would not infringe privately owned
rights. Reference herein to any specific commercial product, process or
service by trade name, trademark, manufacturer, or otherwise does not
necessarily constitute or imply its endorsement, recommendation, or favoring by
the United States Government or any agency thereof. The views and opinions of
authors expressed herein do not necessarily state or reflect those of the
United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available to DOE and DOE Contractors from the Office of Scientific and
Technical Information, P. O. Box 62 Oak Ridge, TN 37831; prices available from
(423) 576-8401.
Available to the public from the National Technical Information
Service, U.S. Department of Commerce, 5285 Port Royal Road, Springfield, VA
22161.
Abstract
A batch vitrification process, which utilizes an oxalate precipitate and frit
(or cullet), is being developed at the Savannah River Technology Center (SRTC)
to immobilize an Am-Cm solution. Prior to being accepted as the baseline
flowsheet, numerous laboratory-scale tests were conducted to demonstrate its
feasibility and to characterize the general melt behavior of the oxalate/frit
system. The effects of frit particle size and oxalate precipitation
temperature were the initial focus of these studies. Two technical issues were
identified during these initial tests that warranted further study: a volume
or bed expansion was observed at approximately 1140°C and "excessive"
bubble formation between 1220 - 1250°C. Although high temperature bubble
formation does not pose a serious process concern (i.e., longer residence times
and/or higher process temperatures minimize bubble retention), the volume
expansion is undesirable during processing. The volume expansion may limit the
amount of glass that can be produced in a single batch. That is, the batch
height may have to be controlled so that the material is contained within the
Pt-Rh vessel at all times.
Both the volume expansion and high temperature bubble formation have been
linked to the thermal reduction of CeO2. As part of the oxalate
feed, Ce is reduced (3+ state). Upon thermal decomposition of the oxalate
under oxidizing conditions, Ce will oxidize (3+ 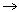 4+ state) which provides
the opportunity for thermal reduction at higher temperatures liberating
O2. Tests using a "Ce-free" oxalate have been performed in which no
indication of either the volume expansion or high temperature bubble formation
were observed. Complementary studies focused on redox and off-gas related
issues provided a fundamental understanding of the melting behavior of the
oxalate/frit system and lead to the successful development of the batch
vitrification process.
4+ state) which provides
the opportunity for thermal reduction at higher temperatures liberating
O2. Tests using a "Ce-free" oxalate have been performed in which no
indication of either the volume expansion or high temperature bubble formation
were observed. Complementary studies focused on redox and off-gas related
issues provided a fundamental understanding of the melting behavior of the
oxalate/frit system and lead to the successful development of the batch
vitrification process.
Introduction
Approximately 11,000 L of solution containing isotopes of Am and Cm, currently
stored at the Savannah River Site (SRS), will be converted into a
high-lanthanide glass for stabilization [1]. Pretreatment operations will be
performed to separate actinides and lanthanides from impurities (primarily
iron, aluminum, and sodium) and to concentrate the solution to approximately
100 g/L dissolved solids (as equivalent metal oxide) in a nitric acid solution.
Approximately 10 wt.% of the total dissolved solids are Am and Cm. The nitric
acid-based Am-Cm solution (or in the case of this study the non-radioactive
surrogate) is transferred into a reaction vessel. Oxalic acid (8 wt.%) is
added to the solution to precipitate the rare-earth elements as oxalates. The
precipitate is allowed to settle and the excess liquid is decanted. A dilute
oxalic acid wash solution (0.10M) is added to remove excess contaminants (e.g.,
Fe, Cr) and reduce the acidity of the feed [2]. The precipitate is again
allowed to settle and the washwater is decanted. The settled slurry is mixed
and then gravity fed to a cylindrical Pt-Rh induction heated vessel. The
proper amount of frit is added to result in a final glass composition
containing ~35 - 45 wt.% of oxides from the Am-Cm solution. A simplified
schematic of the batch process and equipment is shown in Figure 1.
Figure 1. Schematic diagram of the Am/Cm vitrification
process.
The oxalate precipitate slurry and frit are added to the melter vessel and
heated to approximately 100°C. This temperature is held until the mixture
has dried. The vessel temperature is then raised at a rate between 4 and
8°C/min to a glass temperature between 1350 and 1450°C. During this
temperature increase, the remaining nitrate is decomposed and the oxalate
precipitate decomposes to the associated rare-earth oxides. The melt is held
at temperature for approximately 1 to 4 h to ensure adequate mixing of frit and
oxides and is drained from the bottom of the melter by gravity through a drain
tube into a stainless steel canister.
The reaction chemistry involved with converting these oxalate feed stocks into
glass products determines the potential for volume expansion, bubbling, and
other melt and off-gas related phenomena associated with this process.
Knowledge of the chemical processes is critical to vitrification plant design,
operation, and troubleshooting. The rare earth and actinide oxalates will
decompose and release CO and CO2. Residual nitrates and waters of
hydration will also be released during the melting process. Oxidation states
will likely change, and ultimately rare earth and actinide elements will be
incorporated into the glass. Vienna et al. provide a detailed discussion of
the reaction pathways for this system [3].
Prior to accepting this batch process as the baseline flowsheet, numerous
laboratory-scale tests were conducted to demonstrate its feasibility and to
characterize the general melt behavior of the oxalate/frit system. The effects
of frit particle size and oxalate precipitation temperature were the initial
focus of these studies. This report discusses the results of these initial
studies that were performed to evaluate the feasibility of the oxalate-based
batch process. Identification of the mechanism(s) and source(s) of the volume
expansion and high temperature bubble formation was a primary objective.
Complementary studies focused on redox and off-gas related issues provided a
fundamental understanding of the melting behavior of the oxalate/frit system.
Experimental
Initial laboratory-scale tests (i.e., Runs A - G) focused on the effects of the
particle size (-80,+200 mesh, -14,+30 mesh, and cullet) of the 25SrABS
frit (25SrABS is the baseline frit composition established for the Am-Cm vitrification program. This frit contains 25 mass %
La2O3 but has a high solubility limit of other lanthanide
oxides or rare earth oxides. Total lanthanide oxide contents of up to 60% have
been demonstrated while still meeting both process and product performance
constraints.[4]) and oxalate precipitation
temperature (50° and 90°C). Twenty-five (25) grams of oxalate
precipitate was added to 23.52 grams of 25SrABS frit (1.0623 gram of oxalate
per 1 gram of frit) which targets an approximate 39 wt.% feed loaded glass on
an oxide basis. To evaluate the effect of frit particle size and oxalate
precipitate temperature various combinations were utilized (see Table I). The
various oxalate precipitate and pre-sized frit combinations were subsequently
mixed and placed in a 100 ml 95% Pt / 5% Rh crucible. The batches were heated
at 4°C/min up to 1350°C. Characteristics of the melting behavior of
each batch were recorded during the melt regime. After a 0.5 hour isothermal
hold at 1350°C, the crucibles were removed and allowed to cool to room
temperature. Visual observations of the resultant glass product were also
documented.
Results
Visual observations of Runs A - G (-80,+200 mesh and -14,+30 mesh 25SrABS frit coupled
with both the 50 and 90°C oxalate precipitate) are summarized in Table I.
Similar melting characteristics were observed regardless of frit particle size
or oxalate precipitate temperature utilized. In general, as the temperature
increased from room temperature to 500°C, the oxalate/frit batches
transitioned from white to a dark green / rust color. The change in batch
color being associated with the decomposition of the oxalate which occurs over
a temperature span of ~400° - 800°C based on DTA/TGA data. A
reduction in batch volume was observed as a result of the oxalate
decomposition. Based on visual observations at 1000°C, there was no
apparent liquid (or glass) phase present. At 1000°C, Runs A - G were
characterized by a "crusty" top surface which visually appeared crystalline.
As temperatures continued to increase, a common denominator for all tests was a
volume expansion at ~1140°C. The onset of the volume expansion was
characterized by a slight increase in batch height in the center of the
crucible which gradually increased (with increased temperature). The volume
expansion was estimated to be an approximate 1 - 2x increase in batch height at
its maximum. The onset of the volume expansion was consistently observed at
~1140°C for each oxalate/frit system. At ~1200°C, an initial liquid
was observed which was followed by a gradual volume reduction. The formation
of the initial liquid (or glass) phase may have occurred at a lower
temperature.
In concert with the formation of the initial liquid phase, bubbles were
observed on the melt surface near the Pt crucible interface between 1200 -
1240°C. As temperatures increased, bubbles accumulated on the melt
surface. At 1350°C, the degree of bubble coverage on the melt surface
varied from run to run. In some cases, bubbles were observed across the entire
melt surface while only a ring of bubbles around the melt/crucible interface
was observed in other runs. Although varying degrees of coverage were
observed, bubble formation was observed in Runs A - G at temperatures in excess
of 1200°C.
After the 0.5 hour isothermal hold at 1350°C, the glasses were homogeneous
with respect to crystallization but the melt surface was typically
characterized by some degree of very small bubbles on the glass surface. It
should be noted that increased residence times and/or increased process
temperature these bubbles have been demonstrated to be very effective in
eliminating these bubbles (i.e., fining).
Table I. Major Visual Observations During Initial Oxalate / Frit Tests.
Run
|
Frit
Size
|
Oxalate
Precipitate
|
Volume
Expansion
|
Bubble
Formation
|
Resulting
Glass
|
A
|
-80,+200
|
50°C
|
1140°C
|
1200°C
|
No
bubbles
|
B
|
-80,+200
|
90°C
|
1140°C
|
1200°C
|
Bubbles
|
C
|
-80,+200
|
90°C
|
1140°C
|
1240°C
|
Very
few bubbles
|
D
|
-80,+200
|
50°C
|
1140°C
|
1280°C
|
No
bubbles
|
E
|
-14,+30
|
90°C
|
1140°C
|
1220°C
|
Bubbles
|
F
|
-14,+30
|
50°C
|
1140°C
|
1220°C
|
Bubbles
|
G
|
-14,+30
|
90°C
|
1140°C
|
1240°C
|
Very
few bubbles
|
Based on these initial tests, no significant differences in the melting
behavior of these oxalate / frit mixtures were observed. The volume expansion
and high temperature bubble formation was consistently observed in systems
containing either size classification (-14,+30 and -80,+200) of the 25SrABS
frit and each oxalate precipitation temperature (50° and 90°C).
To address the effect of a larger frit particle size, "cullet" was produced by
melting 25SrABS frit at 1450°C and pouring into deionized water. This
technique produced cullet which was characterized by a wide range of particle
sizes (the majority exceeding 14 mesh) and distribution. Since the results of
Runs A - G showed no difference between the 50° and 90°C precipitate,
25SrABS cullet was mixed with 50°C oxalate precipitate and processed under
similar conditions (i.e., 4°C/min to 1350°C with a 0.5 isothermal
hold). Table II summarizes the results of the 50°C oxalate / cullet tests
(Runs H - L). The major difference with the increased particle size (or
cullet) is the absence of the volume expansion observed at 1140ºC in the
previous runs (see Table I). Again, the volume expansion is undesirable from a
processing view point because it may limit the amount of glass that can be
produced in a single batch. The minimization (or elimination) of the volume
expansion via the use of larger frit particles (i.e., cullet) is a major
process advantage. Subsequent testing in the Drain Tube Test Stand (DTTS) and
the Cylindrical Induction Melter (CIM) has demonstrated that the use of cullet
eliminates (or minimizes) the volume expansion observed with finer particle
sizes.[2]. High temperature bubble formation was observed between 1228 and
1300°C during all oxalate / cullet tests.
Table II. Major Visual Observations During Oxalate / Cullet Tests.
Run
|
Frit
Size
|
Oxalate
Precipitate
|
Volume
Expansion
|
Bubble
Formation
|
Resulting
Glass
|
H
|
Cullet
|
50°C
|
Not
Observed
|
1300°C
|
No
bubbles
|
I
|
Cullet
|
50°C
|
Not
Observed
|
1228°C
|
Bubbles
|
J
|
Cullet
|
50°C
|
Not
Observed
|
1240°C
|
Very
few bubbles
|
K
|
Cullet
|
50°C
|
Not
Observed
|
1266°C
|
No
bubbles
|
L
|
Cullet
|
50°C
|
Not
Observed
|
Not
recorded
|
No
bubbles
|
Although the use of cullet does minimize (or eliminate) the volume expansion in
this system, the mechanism was still unknown and the reaction pathways were not
well understood. Therefore supplemental tests were performed to provide a
fundamental understanding of the off-gas and redox related issues in an attempt
to isolate the mechanism of the volume expansion and high temperature bubble
formation. With the mechanism known, chemical (e.g., frit composition) and/or
physical changes (e.g., melter design or heating profiles) could be made, if
necessary, to the system and the batch flowsheet could be refined.
Supplemental tests in the development of the batch process included "frit-only"
tests, an evaluate of redox as a function of temperature for select multivalent
cations associated with the feed, gas analysis of samples from a pilot scale
melter, and quartz crucible tests that characterized both off-gas as a function
of temperature and confirmed of visual observations of the initial
laboratory-scale tests. The results of each of these complementary tests can
be used as part of the larger body of data to support the evaluation of the
batch process as a viable flowsheet option and also provides critical data to
establish a fundamental understanding of the reaction kinetics and phenomena
occurring in this system.
Frit-Only Tests
The objective of the "frit-only" tests was to confirm that the source of the volume
expansion was associated with the feed material; not the 25SrABS frit
composition. These tests utilized both the 25SrABS frit (no surrogate feed
materials included) and pre-fabricated glass compositions (containing all or
part of the surrogate feed components). Two pre-fabricated glasses fabricated
at 1350°C from reagent grade oxides and carbonates were evaluated. The
initial pre-fabricated glass targeted a 30 wt.% feed loading (referred to as
50SrABS Hybrid) which contained all the surrogate components associated with
the oxalate based tests. The second pre-fabricated glass was Frit 1000. This
was a lanthanide-based borosilicate glass containing 6.8 wt% CeO2 as
a surrogate. Pre-sized 25SrABS frit classifications (-14,+30, -80,+200, and
cullet) were also evaluated in the absence of oxalate (i.e., no surrogate
present).
In these tests, approximately 50 grams of pre-fabricated glass or frit was
added to a 100 ml 95% Pt / 5% Rh crucible and heated at 4°C/min to
1350°C or 1450°C (for the 25SrABS frit only). Visual observations
were recorded during the melt regime as well as on the resulting glass product.
Although no volume expansion was observed in any system (consistent with
melting a prefabricated glass or frit), the results were very effective in
isolating the source of the high temperature bubble formation. High
temperature bubble formation was not observed with the 25SrABS frit up to
1450ºC. However, tests with 50SrABS Hybrid and Frit 1000 resulted in the
formation of high temperature bubbles at 1220°C and 1250ºC,
respectively. The temperature at which bubbles formed in these tests is very
consistent with those observed in Runs A - L (see Tables I and II). The initial
hypothesis for the source of bubble formation was thermal reduction of one (or
more) of the multivalent oxides associated with the surrogate. A primary
candidate was Ce (present in both 50SrABS Hybrid and Frit 1000) which is known
to thermally reduce at high temperatures. This hypothesis was confirmed via
subsequent testing.
Redox Analyses
To provide insight into Ce redox (Ce4+/Ce3+) as a
function of temperature, samples of the oxalate precipitate were heated at
110°C for 24 hours, 500°C (DTA/TGA data indicates the onset of
oxalate decomposition at ~400°C) for 2 hours, and 1300°C for 24 hours
under oxidizing conditions. The samples were dissolved in a mixture of
hydrofluoric and sulfuric acid at room temperature and an aliquot portion of
the stock solution was titrated by
(NH4)2Fe(SO4)2 at potentiometric
indication of the equivalence point. A calibration curve of Ce4+
was established by using a standard ceric sulfate solution. Ce3+
was determined by difference using the targeted total Ce concentration.[5] The
Ce redox results are summarized in Table III.
The results indicate that Ce, starting almost completely reduced in the oxalate
form (Ce4+/Ce3+ = 0.01), oxidized partially
(Ce4+/Ce3+ = 0.55) when heat treated to 500°C, and
completely reduced (Ce4+/Ce3+ = 0.00) when heat treated
to 1300°C. These data are consistent with DTA/TGA data that indicate
oxalate decomposition occurring between 400° - 600°C. The oxidation
of cerium upon thermal decomposition provides the opportunity for thermal
reduction at higher temperatures. The redox analyses indicate full reduction
of cerium by 1300°C, which supports the high temperature bubble formation
observed in all systems containing the surrogate (i.e., thermal reduction
provides a gas source (O2) for bubble generation).
Table III. Ce Redox as a Function of Temperature in the Oxalate
Precipitate.
Sample
|
Ce4+
Measured
|
[Ce4+
]/[Ce3+ ]
|
110°C,
24 hours
|
0.30
|
0.01
|
500°C,
2 hours
|
10.52
|
0.55
|
1300°C,
24 hours
|
0.00
|
0.00
|
Gas Analysis
Two Drain Tube Test Stand (DTTS, a 2.5 " cylindrical pilot scale Pt melter)
samples were collected from the melt surface during the vitrification of an
oxalate / cullet mixture at approximately 1200°C. The samples were
visually characterized as a frothy or foamy glass (i.e., glassy but containing
numerous small bubbles). These samples were sent to Corning Engineering
Laboratory Services (CELS) for gas analysis by mass spectroscopy via the "crack
method". Results of the bubble analyses indicated that the bubbles were
"oxygen-rich": 70±5% O2, 3±1% CO2, 27±4%
N2 and/or CO (the mass spectrometer used for this analysis could not
distinguish between N2 and CO, which both have a mass of 28 amu).
Since at room temperature virtually no CO is in equilibrium with 70%
O2, it is likely that a majority of the mass 28 species is
N2. The present of "oxygen-rich" bubbles in the DTTS samples
supports the theory of CeO2 reduction leading to O2
generation which results in the high temperature bubble formation.
Quartz Crucible Furnace Experiments
The reaction chemistry involved with converting these oxalate feed stocks into
glass products determines the potential for volume expansion, bubbling, and
other melt and off-gas related phenomena associated with this process.
Although the source of the high temperature bubble formation has been linked to
the thermal reduction of CeO2, the volume expansion observed in the
initial tests at 1140°C (see Table I) have not. The volume expansion
could be related to CeO2 reduction and/or other off-gas related
issues. To gain some understanding of the off-gas generated during oxalate
decomposition, calcination, and the vitrification processes, the PNNL quartz
crucible (QC) furnace was utilized. This furnace allows for visual
observations of the batch during the batch-to-glass conversion process as well
as "real-time" monitoring off-gas as a function of time (which can then be
correlated to temperature) via a GC/MS.[6] Initially, three oxlalate / frit
batches were initially tested using the quartz crucible furnace. These initial
QC tests utilized a -80,+200 mesh 25SrABS frit with a dried 50°C oxalate
precipitate targeting 39 wt% feed loading (on an oxide basis). The batches
were heated at approximately 4°C/min to 1350°C. Based on previous
tests, the use of the -80,+200 mesh particle size should result in a volume
expansion at approximately 1140°C. Table IV shows the major run conditions
and observations.
Table IV. Run Conditions and Major Observations of PNNL Quartz Crucible
Tests.
Run
|
Atmosphere
|
Frit
Size
|
Initial
Liquid
|
Volume
Expansion
|
Bubble
Formation
|
1017
|
Inert
|
-80,+200
|
1110°C
|
Not
observed
|
Not
observed
|
1018
|
Inert
|
-80,+200
|
1150°C
|
Not
observed
|
Not
observed
|
1018
O2
|
O2
|
-80,+200
|
1075°C
|
1075°C
|
1216°C
|
Runs 1017 and 1018 were performed under inert conditions (He / 2% Ar) in an
effort of to monitor O2 liberation (from CeO2 reduction)
as a function of time (or temperature). The intent was to link the on-set of
CeO2 reduction to the volume expansion via the detection of
O2. Although an initial liquid (or glass) phase was observed at
1110° and 1150°C for Runs 1017 and 1018, respectively, there was no
evidence of a volume expansion, high temperature bubble formation, or
O2 detection. This was initially surprising since all previous runs
with the -80,+200 mesh frit resulted in both phenomena. Coupling the Ce redox
data (see Table III) with the results of Runs 1017 and 1018 under inert
conditions, the absence of the volume expansion and high temperature bubble
formation are easily explained. As part of the oxalate feed, Ce is reduced (3+
state). Upon thermal decomposition of the oxalate (onset at ~400°C) under
oxidizing conditions, the Ce will oxidize (3+  4+ state) and then
thermally reduce at high temperatures. Under inert conditions (He / 2% Ar), Ce
will remain in the 3+ state and thus there is no thermal reduction at high
temperatures and hence no source for the volume expansion or high temperature
bubble formation. Runs 1017 and 1018 confirm that the volume expansion and
high temperature bubble formation is a result of the thermal reduction of
CeO2. It should be noted the detection of the initial liquid phase
in Runs 1017 and 1018 at 1110 and 1150°C, respectively, is consistent with
the onset of the volume expansion observed in Runs A - G.
4+ state) and then
thermally reduce at high temperatures. Under inert conditions (He / 2% Ar), Ce
will remain in the 3+ state and thus there is no thermal reduction at high
temperatures and hence no source for the volume expansion or high temperature
bubble formation. Runs 1017 and 1018 confirm that the volume expansion and
high temperature bubble formation is a result of the thermal reduction of
CeO2. It should be noted the detection of the initial liquid phase
in Runs 1017 and 1018 at 1110 and 1150°C, respectively, is consistent with
the onset of the volume expansion observed in Runs A - G.
Observations during Run 1018 O2 (flowing He/21% O2)
indicated a large batch expansion (~ 3× volume) starting at 1075°C
and peaking at roughly 1150°C before subsiding. Additionally, bubbles
formed in the melt from 1200°C to 1240°C. These quartz crucible
furnace observations agree with those made during initial laboratory-scale (see
Table I) and pilot plant tests [2], suggesting that the roughly 5 g quartz
crucible tests are a reasonable simulation of pilot scale melting. Tests using
a "Ce-free" oxalate have been performed in the DTTS and CIM. The results
showed no indication of either the volume expansion or high temperature bubble
formation that confirm that cerium reduction is the primary source for both
phenomena. Vienna et al. provide a detailed discussion of the reaction
pathways for this system [3].
The proposed mechanism for the volume expansion has been linked to the overlap
of two key processes: the softening of the 25SrABS frit and the release of
O2 via the thermal reduction of multivalent oxides in the feed. As
temperatures increase in the oxalate / frit system, thermal decomposition
occurs at approximately 500ºC. Under oxidizing atmospheres, the
multivalent oxides (reduced in the oxalate) oxidize creating the potential for
thermal reduction at higher temperatures. At approximately 1140ºC, the
25SrABS frit beings to soften while thermal reduction of the oxidized
multivalent oxides is occurring. With the softening of the 25SrABS frit, a
highly viscous liquid is formed which traps the O2 being liberated
from thermal reduction. The result is an increase in batch volume until
sufficient liquid phase is formed with a viscosity that allows for the trapped
bubbles to escape. Once a liquid phase, bubble formation can still be observed
until the thermal reduction of the multivalent oxides is complete
(approximately 1300ºC in this system). Again, increased residence times
and/or processing temperatures have shown to be effective in eliminating bubble
from the final product.
Summary
A batch vitrification process, which utilizes an oxalate precipitate and frit (or
cullet) is being developed at the Savannah River Technology Center (SRTC) to
immobilize an Am-Cm solution. Prior to being accepted as the baseline
flowsheet, numerous laboratory-scale tests were conducted to demonstrate its
feasibility and to characterize the general melt behavior of the frit/oxalate
system. The effects of frit particle size and oxalate precipitation
temperature were the initial focus of these studies. The results of these
initial tests indicated no significant difference in the melting behavior of
batches using various combinations of -80,+200 or -14,+30 mesh 25SrABS frit
coupled with 50° or 90°C oxalate precipitate. Two technical issues
were observed during these initial tests that warranted further study. A
volume or bed expansion was observed at approximately 1140°C and
"excessive" bubble formation between 1220 - 1250°C.
The Ce redox studies indicated that Ce, starting almost completely reduced in
the oxalate form (Ce4+/Ce3+ = 0.01), oxidized partially
(Ce4+/Ce3+ = 0.55) when heat treated to 500°C, and
completely reduced (Ce4+/Ce3+ = 0.00) when heat treated
to 1300°C. These data are consistent with DTA/TGA data that indicate
oxalate decomposition occurring between 400° - 600°C. The oxidation
of cerium upon thermal decomposition provides the opportunity for thermal
reduction at higher temperatures (i.e., thermal reduction provides a gas source
(O2) for bubble generation).
No volume expansion or high temperature bubble formation was observed in the
oxalate / frit quartz crucible tests performed under inert conditions (2% He/
Ar). Under inert conditions (He / 2% Ar), Ce will remain in the 3+ state
throughout the decomposition, calcination, and vitrification processes and thus
there is no thermal reduction at high temperatures. Therefore, the gas source
to cause the volume expansion or high temperature bubble formation is not
present.
Observations during Run 1018 O2 (flowing He/21% O2)
indicated a large batch expansion (~ 3× volume) starting at 1075°C
and peaking at roughly 1150°C before subsiding. Additionally, bubbles
formed in the melt from 1200°C to 1240°C. These quartz crucible
furnace observations agree with those made during initial laboratory-scale and
pilot plant tests [2], suggesting that the roughly 5 g quartz crucible tests
are a reasonable simulation of pilot scale melting. Tests using a "Ce-free"
oxalate have been performed in the DTTS and CIM. The results showed no
indication of either the volume expansion or high temperature bubble formation
that confirm that cerium reduction is the primary source for both phenomena
Laboratory, pilot, and full-scale testing has demonstrated that the volume
expansion can be minimized (or eliminated) through the use of large particle
sized frit (i.e., 25SrABS cullet). Tests using a "Ce-free" oxalate have been
performed in the DTTS and CIM. The results showed no indication of either the
volume expansion or high temperature bubble formation that confirm that cerium
reduction is the primary source for both phenomena.
References
- J. E. Marra, M. A. Baich, A. P. Fellinger, B. J. Hardy, T. M. Jones, C. B.
Miller, D. H. Miller, D. K. Peeler, T. K. Snyder, M. E. Stone, J. C.
Whitehouse, and D. C. Witt, "Americium-Curium Vitrification Pilot Tests - Part
II," in Proceedings of the International Symposium on Waste Management
Technologies in Ceramic and Nuclear Industries, Ceramic Transaction, Vol.
89, Am. Ceram. Soc., Westerville, OH (1999).
- A. P. Fellinger, M. A. Baich, B. J. Hardy, G. T. Jannik, T. M. Jones, J. E. Marra,
C. B. Miller, D. H. Miller, D. K. Peeler, T. K. Snyder, M. E. Stone, and D. C.
Witt, "Americium/Curium Vitrification Process Development," to be published in
the proceedings of Mater. Res. Soc., Pittsburgh, PA (1999).
- J. D. Vienna, D.K. Peeler, J.G. Darab, J.R. Zamecnik, H. Li, and J.E. Marra,
"Chemistry of Rare Earth Oxalate Vitrification," to be published in the
proceedings of Mater. Res. Soc., Pittsburgh, PA (1999).
- D.K. Peeler, T.B. Edwards, I.A. Reamer, J.D. Vienna, D.E. Smith, M.J.
Schweiger, B.J. Riley, and J.V. Crum, "Composition / Property
Relationships for the Phase 1 Am/Cm Glass Variability Study (U)",
WSRC-TR-99-00055, Rev. 0, Westinghouse Savannah River Company, Aiken, SC
1999.
- H. Li, J.D. Vienna, P. Hrma, M.J. Schwieger, D.E. Smith, and M. Gong,
"Borosilicate Based Glasses for Immobilization of Plutonium-Bearing Materials,
Ceramic Transactions, Volume 72, pp. 399 - 408 (1996).
- P.A. Smith,, J.D. Vienna, and P. Hrma, "The Effect of Melting Reactions on
Laboratory Scale Waste Vitrification," J. Mater. Res, Vol. 10 (1995).
 4+ state) which provides
the opportunity for thermal reduction at higher temperatures liberating
O2. Tests using a "Ce-free" oxalate have been performed in which no
indication of either the volume expansion or high temperature bubble formation
were observed. Complementary studies focused on redox and off-gas related
issues provided a fundamental understanding of the melting behavior of the
oxalate/frit system and lead to the successful development of the batch
vitrification process.
4+ state) which provides
the opportunity for thermal reduction at higher temperatures liberating
O2. Tests using a "Ce-free" oxalate have been performed in which no
indication of either the volume expansion or high temperature bubble formation
were observed. Complementary studies focused on redox and off-gas related
issues provided a fundamental understanding of the melting behavior of the
oxalate/frit system and lead to the successful development of the batch
vitrification process.