
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available to DOE and DOE Contractors from the Office of Scientific and
Technical Information, P. O. Box 62 Oak Ridge, TN 37831; prices available from
(423) 576-8401. Available to the public from the National Technical Information
Service, U.S. Department of Commerce, 5285 Port Royal Road, Springfield, VA
22161.
The Department of Energy (DOE) plans to immobilize at least some of the excess weapons useable plutonium in a ceramic form for final geologic disposal. The proposed immobilization form is a titanate based ceramic consisting primarily of a pyrochlore phase with lesser amounts of brannerite, rutile, perovskite, zirconolite and/or glassy phases. The ceramic formulation is cold-pressed and then densified via a reactive sintering process. The sintering process results in approximately 20% shrinkage from the green state. The final phase assemblage appears to be the result of several reactions occurring during the reactive sintering process. In this study, thermal analysis techniques were coupled with x-ray diffraction analysis (XRD) in an attempt to identify reaction temperatures and mechanisms occurring during the heating process. Several low temperature reactions involving calcium in various chemical states were identified. The in-growth of perovskite was also pinpointed as well as the development of the primary phase - pyrochlore. The formation of pyrochlore in the ceramic was found to coincide with the onset of densification.
In the aftermath of the Cold War, approximately 50 tonnes (MT) of weapons useable Pu has been identified as excess. The U.S. Department of Energy (DOE) has decided that at least a portion of this material will be immobilized in a titanate-based ceramic for final disposal in a geologic repository [1]. The technology to be employed will involve immobilizing the Pu in ceramic "pucks", placing the pucks in cans and then encasing these cans in radioactive high-level waste glass (i.e. can-in-canister) [2]. The can-in-canister configuration, including the high radiation field afforded by the waste glass, will provide the necessary proliferation resistance.
The proposed immobilization formulation contains chemical precursors to produce a durable ceramic form as well as sufficient neutron absorbers to preclude criticality. The baseline formulation shown in Table I was designed to produce a ceramic consisting primarily of a highly substituted pyrochlore with minor amounts of brannerite and hafnia-substituted rutile. Many of the prospective feed streams contain large amounts of uranium in addition to plutonium. The pyrochlore phase was shown to readily accommodate large quantities of actinides [2]. In addition, given that pyrochlore has a cubic structure, it is anticipated that the isotropic nature of the mineral phase will minimize radiation damage. When impurities are present in the Pu feed streams other minor phases such as zirconolite, perovskite, glassy phases, etc. may be present in the ceramic form. However, the composition was designed such that all of the phases that contain actinides also have significant amounts of neutron absorbers. Chemical formulas (showing the atomic substitutions) for the various mineral phases observed in the ceramic are shown in Table II.

Concurrently with formulation development efforts, a ceramic fabrication process is being developed based on cold pressing and sintering. In summary, the current baseline process consists of:
* milling the actinide oxide feeds to less than 20 µm in an attritor
mill
* mixing/milling the milled actinide oxides with a commercially supplied
precursor batch in a second attritor mill
* granulating the conditioned powders
* cold pressing the granulated powders into "pucks"
* sintering the pressed "pucks."
Nominal sintering conditions have been established for the ceramic form consisting of heating at 3° C/min to 300° C, 2 hour hold at 300° C for binder burnout, 5° C to 1350° C, hold at 1350° C for 4 hours, and cooling at 5° C/min to room temperature [3]. This sintering schedule is subject to change, however, as the fabrication process matures. The objective of the sintering process is to produce a monolithic product with a phase assemblage that will ensure product durability. Unlike solid state or single phase sintering processes, the sintering of the Pu immobilized ceramic form is "reactive" in nature, where the oxide precursors react to form highly substituted mineral phases. To date, little is known about the reactions that occur and their progression. The objective of this study was to identify reactions and reaction temperatures occurring in the ceramic formulation upon heating in hopes of better understanding the phase development progression in the ceramic.
Several experimental techniques were used in this study. Initially, differential thermal analysis (DTA) and thermogravimetric analysis (TGA) were used to pinpoint potential reaction temperatures. From these results, samples were subjected to isothermal heat treatments at temperatures before and after detected reactions deduced from the DTA/TGA results. These samples were then analyzed using x-ray diffraction to determine the phase assemblage. Following this initial testing, more refined analyses were made on selected individual batch components and the baseline composition using high temperature x-ray diffraction and thermal analysis techniques.
In all testing, cerium oxide was substituted on a molar basis for the actinide oxides in the baseline formulation. Although the use of cerium as a surrogate greatly facilitates testing, the tendency of cerium to change oxidation states complicates phase development. When cerium is used in the baseline formulation, the perovskite concentration is enriched while the brannerite concentration is nonexistent. It is anticipated that much of this work will be repeated in the near future using the actinide bearing composition.
The precursors listed in Table I with CeO2 as a surrogate for PuO2 and UO2 on a one to one molar basis were weighed to form a 100 g batch. The batch was added to a polyethylene bottle filled approximately half full with 1/8 inch spherical zirconia grinding media. Deionized water was added to just cover the batch and grinding media. The mixture was then ball milled for 22 hours. The milled slurry was poured through a #20 sieve opening to catch the grinding media and allow the slurry to collect in a drying pan. The solution was dried for 48 hours at 105° C. The dried material was removed from the pan and stored for later testing. In the preliminary, baseline production process, the precursors will be calcined at 750° C. At the time of this study, the calcining protocol was not established, hence the batch materials were not calcined.
Differential thermal analysis and TGA were run (in duplicate) on samples of the batch to identify reaction temperatures. Typical scan rates of 10° C/min were used in all of the testing. The DTA scans were run to the nominal sintering temperature of 1350° C. Due to instrument limitations the TGA scans were run only to 1200° C (it should be noted that no weight loss was detected beyond about 750° C). The results were also evaluated using the first derivative to aid in the identification of reactions. Once potential reaction temperatures were determined, a series of isothermal heat treatments at temperatures slightly above those depicted in the thermal analysis scans were run on the material. Approximately 10 g of material was heated in an alumina crucible for four hours at the selected temperature and then air quenched to room temperature. The heat treated samples were carefully divided to remove any material that may have interacted with the crucible. X-ray diffraction was run on all heat treated samples to determine the phases present.
The objectives of the high temperature x-ray diffraction studies were to analyze reactions "in-situ" to better quantify reaction temperatures and to gain insight into the reaction kinetics. The baseline ceramic composition and a few individual batch components were tested. As will be discussed later, reactions upon heating involving Ca(OH)2 and CaCO3 were of particular interest so reagent grade chemicals of these compounds were also tested individually.
In the high temperature XRD experiments, a small amount of material was placed on a platinum ribbon and placed in the diffractometer. The ribbon was rapidly heated to the desired temperature and held at that temperature for times up to several hours. The detector on the diffractometer was set to an angle corresponding to a distinct diffraction peak for the tested material and the intensity of the peak was recorded as a function of time.
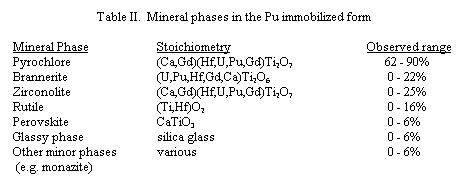
The initial DTA and TGA scans revealed potential endothermic reactions or "trends" in the following estimated ranges: 40-150° C; 325-370° C; 385-475° C; 540-725° C; 945-990° C; and 1230-1350° C. It should be noted that the last endothermic trend was not "completed" at 1350° C. Several weight changes were observed to correspond with these reaction temperatures. Weight losses were seen in the following temperature ranges: 90-120° C (0.2 wt %); 240-275° C (0.2 wt %); 300-400° C (2 wt %); 520-680° C (1.6 wt %). A small weight gain (0.2 wt %) was also observed between 400 and 520° C.
Based on these results, isothermal heat treatments were conducted on samples at 375, 400, 550, 600, 700, 950 and 1130° C. The phase assemblage of the heat treated samples were then determined by XRD in an attempt to quantify the reaction mechanisms occurring in the various temperature regions. The XRD results are summarized in Table III.
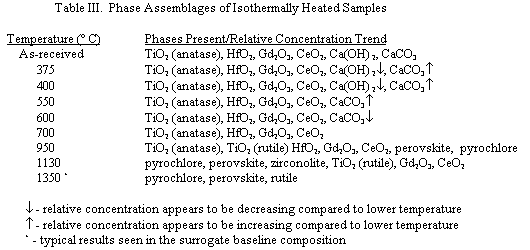
The isothermal heat treatment studies combined with the thermal analysis results yielded several interesting observations. The XRD scan for the as-received material indicated that carbon dioxide (present in the air) reacts with the calcium hydroxide during processing and powder storage to form a calcium carbonate phase in the starting material. Upon heating, the calcium hydroxide phase began to decompose at temperatures as low as 240° C (TGA weight loss data) and was completely removed from the batch by 550° C (XRD data). From the XRD data, it was also evident that calcium carbonate "grows-in" during the initial stages of heating. This finding was further validated by the weight gain observed in the TGA scans. The decomposition reaction involving calcium carbonate began near 550° C and was completed by 700° C. Immediately after the decomposition of the calcium hydroxide and calcium carbonate (~700° C) there was no "calcium compound" evident in the XRD scan indicating that the calcium compounds decomposed into very fine CaO crystallites. Upon heating to 950° C, both perovskite and pyrochlore began to form in the ceramic. Upon further heating, the pyrochlore phase became predominant.
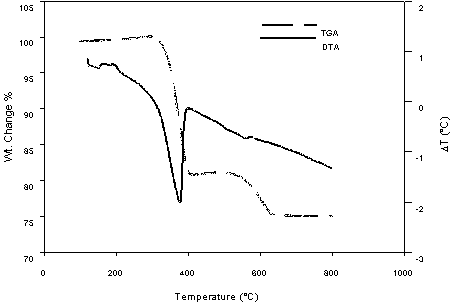
In an effort to better understand the observations described above, additional testing using thermal analysis and high temperature XRD was performed on individual batch components and the baseline composition. Both DTA and TGA were performed on reagent grade calcium hydroxide (Figure 1). This testing confirmed the observed behavior of calcium hydroxide in the batch materials (i.e. decomposition of Ca(OH) 2, in-growth of CaCO3, decomposition of CaCO3).
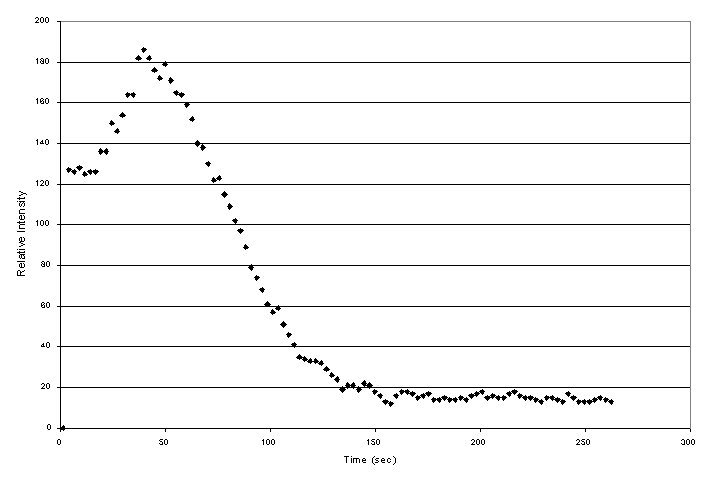
High temperature XRD testing on reagent grade Ca(OH)2 also confirmed the behavior upon heating of calcium hydroxide in the baseline composition. Figure 2 shows the decrease in relative intensity of the major Ca(OH)2 diffraction peak as a function of time for a sample heated constantly at 400° C.
The in-growth of calcium carbonate on Ca(OH)2 was observed in-situ by heating reagent grade Ca(OH)2 at 450° C and observing an increase in intensity in the major CaCO3 diffraction peak (Figure 3).
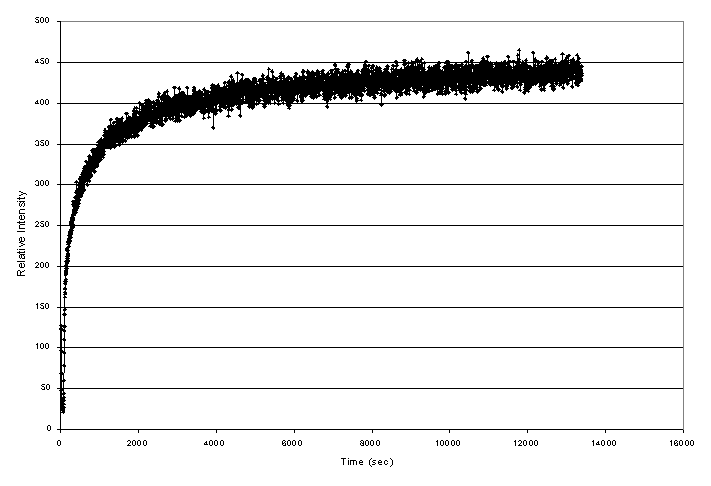
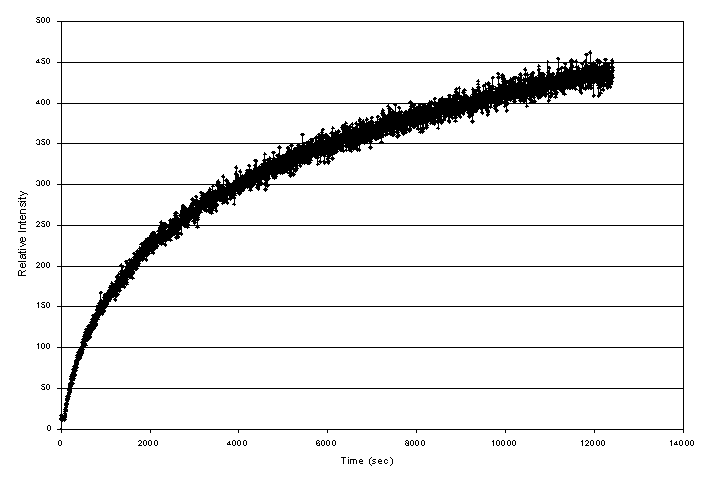
High temperature XRD performed on the surrogate baseline composition also provided insight into ceramic phase formation. In separate experiments, a sample of the baseline composition was rapidly heated to 1000° C and the formation of perovskite and pyrochlore was monitored by examining a distinct peak of the respective phase. The perovskite was observed to rapidly form in the material to an apparent saturation concentration (Figure 4). In contrast, the pyrochlore phase appeared to form gradually and increased in concentration with time at 1000° C (Figure 5). The differences in the observed growth of these two phases in the high temperature XRD studies combined with the isothermal heat treatment studies led to the following hypotheses. The perovskite formed initially in the ceramic and reached a saturation concentration upon further heating. The perovskite may have acted as a precursor for pyrochlore development. Once the pyrochlore phase development was initiated the chemical driving forces in the system acted to form the predominant pyrochlore phase upon continued heating.

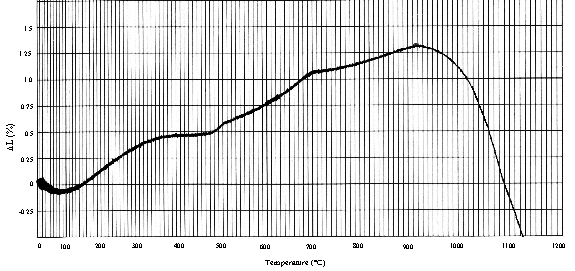
The formation of the pyrochlore phase was also observed to coincide with the densification of the ceramic. Dilatometry scans have shown that the densification of the ceramic begins at approximately 920° C and continues quite rapidly upon further heating (Figure 6). It is also interesting to note in the dilatometry curve the apparent increase in expansion from approximately 280 to 450° C. This temperature range roughly coincides with the decomposition of Ca(OH)2 and in-growth of CaCO3 reactions observed in the thermal analysis and XRD studies. The sample material tested by dilatometry was calcined at 750° C prior to testing. However, XRD results on the calcined powder showed that most of the calcium was present in the form of Ca(OH)2 and CaCO3 after calcination and storage.

The information contained in this paper was developed during the course of work under Contract No. DE-AC09-96SR18500 with the U.S. Department of Energy.