WSRC-MS-2002-00086
Improved Isotopic Measurement of Plutonium by
Thermal Ionization Mass Spectrometry
P. Cable-Dunlap, D. Fauth, G. Hall, S. P. LaMont,
L. S. Nichols, and C. R. Shick, Jr.
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/index.html
Available electronically at http://www.osti.gov/bridge
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Introduction
Thermal ionization mass spectrometry (TIMS) is accepted widely as the benchmark method for precise and accurate isotopic determination of plutonium. TIMS is one of the few analytical methods capable of determining Pu in bioassay samples at the level required for detecting a 50 yr committed dose of 100 mRem resulting from an inhalation exposure to highly insoluble forms of Pu. Typically, Pu is measured in bioassay samples by radiochemical separation, electrodeposition onto a planchet, and radiometric determination by alpha spectrometry. If, based on the alpha spectrometry results, a sample is deemed to need a more sensitive analysis (i.e., suspected uptake, borderline alpha spectrometry positive for Pu uptake, etc.), then the sample is prepared for analysis by TIMS.
Part of the development process for establishing a program to determine Pu in bioassay samples by TIMS at the Savannah River Site involved a careful evaluation of the Pu blank value in the reagents used for sample preparation and in urine blanks. This exercise allowed for the evaluation of the newly developed radiochemical separation procedure, the resin bead loading procedure, and the detection limits of the thermal ionization mass spectrometer.
Experimental
A batch of 10 reagent blanks and a batch of 10 urine blanks (500 mL) were prepared and chemically purified using the standard SRS Bioassay radiochemical separation procedures. The blank samples were then analyzed by alpha spectrometry. The blanks were spiked with two different tracers, 1.25 dpm of 236Pu as an alpha spectrometry tracer, and 5.2 pg of 242Pu as a TIMS tracer. Only ultra-pure reagents were used for all chemical processing of samples after alpha spectrometry and prior to analysis by TIMS. After the initial chemical yield determination by alpha spectrometry, the Pu was redissolved off the planchets by leaching overnight in 3 mL of 8 M HNO3. This solution was transferred to a 3 mL TeflonÔ conical vial, taken to dryness under a heatlamp, then reconstituted in 0.5 mL 8 M HNO3.
A 0.20 mL AG 1 x 4 Cl- form anion exchange column was used to purify the Pu. This column was prepared by passing 1 mL of 0.1 M HCl over it to rinse out any impurities and followed by 1 mL of 8 M HNO3. The sample was then added to the column, and the conical vial was rinsed into the column with two 0.5 mL volumes of 8 M HNO3. The column was then rinsed with an additional 0.5 mL of 8 M HNO3, to remove any trace uranium impurities, and then with 1 mL of 9 M HCl to remove any trace thorium impurities (which greatly decrease the ionization efficiency for Pu). Finally, Pu was eluted into a clean 5 mL conical Teflon vial with 0.5 mL concentrated HBr, then taken to dryness under a heatlamp. The sample was wet ashed twice with 0.5 mL 8 M HNO3, and then reconstituted in 5 m L 8 M HNO3.
Four 50 – 100 mesh AG 1 x 4 Cl- form anion exchange beads were added to the sample and the vial was placed on an orbital shaker for 4 hours. The beads were then removed from the sample solution with a tungsten needle and placed in the center of a V-shaped TIMS filament. A drop of collodion was added to the filament to fix the resin beads. The filaments were then loaded into a pyrolization chamber and heated to approximately 1400°C to burn off most of the organic material present in the resin beads. Finally, the filaments were loaded into the TIMS instrument and slowly heated to approximately 1650°C. The instrument was tuned on 242Pu, and the concentration was calculated by taking the measured ratio of the atomic percents and subtracting the ratio of the NIST values for the 242Pu spike. The resultant value was then multiplied by the amount of the 242Pu spike added to the original sample and divided by the amount of sample processed.
Results
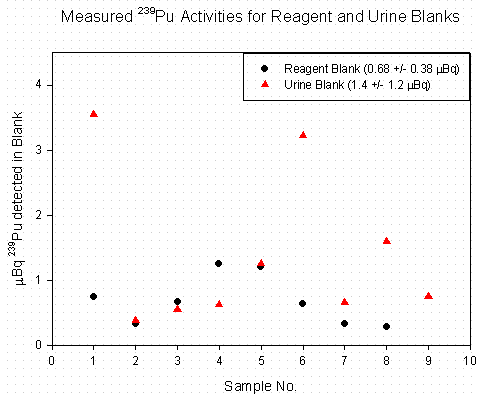
Eight of the 10 reagent blank samples and 9 of the 10 urine blank samples produced good 242Pu signals. A post-run inspection of the filaments that did not produce sufficient signal revealed that the resin beads were lost in the preheating process. The 239Pu concentrations measured in the reagent blanks and urine blanks are shown in Figure 1. As can be seen in the data, the range of blank values in the urine blanks is much larger than the range in the reagent blanks. A rough detection limit (Ld) of 3.9 m Bq was calculated for 239Pu in urine by taking 3.29 times the standard deviation in the measured 239Pu value in the urine blanks. While this is only an estimation of the Ld, with the limited data set, it is appropriate and shows that the sample preparation and TIMS analysis methods are good enough to determine Pu in urine at the required levels. A more rigorous statistical evaluation of the blank will be done when there is a larger data set.

Figure 1. Background 239Pu Levels in Reagent and
Urine Blanks