WSRC-MS-2001-00396
Catalysis of Tetraphenylboron by Pd Nanoclusters in the Presence of Hg
M. C. Duff, D. B. Hunter, M. J. Barnes, R. A. Peterson, and S. D. Fink
Westinghouse Savannah River Company
Aiken, SC 29808
J. P. Bradley
MVA Incorporated
Norcross, GA 30093
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department
of Energy and its contractors, in paper, from: U.S. Department of Energy, Office
of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062,
phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
More than 300-million L of high-level radioactive waste (HLW), a fission-product material associated with the dissolution of spent nuclear fuel for recovery of plutonium, await treatment at U.S. Dept. of Energy (DOE) sites. Previous attempts to concentrate radiocesium by precipitation with tetraphenylborate (TPB) produced unexpectedly high levels of benzene (a TPB decomposition product). The waste contains noble metal catalysts but elevated levels of Hg in the waste could inhibit catalysis. Our studies with simulated HLW shows Hg enhances the Pd-catalyzed decomposition of TPB. After reaction, Pd exists as 3 to 10 nm nanoclusters and a PdHg amalgam.
The priority environmental problem for the U.S.DOE is the disposal of high-level nuclear waste (HLW). Precipitation of radiocesium with tetraphenylborate (TPB) from HLW (the waste associated with spent fuel dissolution for plutonium recovery) is being considered as a treatment process. Roughly 130 million L of this extremely radioactive waste await treatment at the Savannah River Site (SRS). Previous attempts to treat HLW tanks with TPB at the SRS resulted in the evolution of benzene (a TPB decomposition product) at levels that did not permit the safe operation of the facility at design throughput rates (1). Although low levels of benzene from the radiolytic and thermal decomposition of TPB were anticipated, the benzene levels were underestimated. The benzene likely formed from decomposition by noble metals within the waste.
At the recommendation of the Defense Nuclear Facilities Safety Board (DNFSB), follow-up studies were conducted to obtain an improved understanding about the nature of the benzene generation (2). Tests with HLW tank sludge and TPB slurries resulted in rapid decomposition of TPB after a 2-month induction period (3). Studies with simulated high-pH waste determined the reaction proceeds most readily when the following are present: benzene, one or more TPB decomposition intermediates (tri-, di- and mono-phenylborate), Hg, and noble metals with Pd as the most reactive noble metal (4-5). In decomposition studies with TPB in HLW simulants with low starting ratios of Pd:Hg, near complete loss of Pd from solution occurs during TPB decomposition and a precipitant forms (4-5). Reduction of Pd(II) to Pd(0) in HLW simulants at low redox potentials is favored (6). However, no information on the catalytic Pd forms was known prior to this study.
Catalytic forms of Pd may consist of dissolved Pd(II) complexes, supported Pd (II,0) forms and nanoclusters of Pd(0). Metallic nanoclusters consist of particles <10 nm in diameter, which are often too small to possess the characteristic properties of their bulk materials (7). Hence, most of the atoms are present on the surface and on average, tend to have incomplete coordination shells (7). Due to their small size, catalytic nanoparticles can be highly reactive because their reactivity is a function of their surface area to volume ratio. Nanoparticles can be unstable in solution due to their tendency to coalesce—a process that decreases their surface area to volume ratio. However, nanoparticles can be stabilized against aggregation in solution by three conditions: electrostatically (e.g., by Na+ ions as in concentrated NaOH), by ligands (e.g., organic and inorganic compounds with bulky functional groups) and by a combination of both conditions (e.g., polyoxoanions) (7). Bimetallic nanocluster catalysts can form from metals normally immiscible in the bulk phase—even those known to be catalytically inert such as Au (8-10). The presence of a second metal in a nanocluster can enhance catalyst versatility and functionality (8-10).
Aliquots of a Pd(II)-nitrate aqueous solution were added to diphenylmercury (DPM) (11) so that initial solution Pd:Hg mole ratios (MR) were 34 [2 g Pd(II) L-1], 3.4, 1.7 and 0.34. The mixtures were added to 125 mg L-1 each of the TPB decomposition products (tri-, di- and mono-phenylborates), 5.4 g L-1 NaTPB, 0.82 g L-1 benzene, 2 M NaOH, and 1 wt % KTPB (a potential solid support), heated to 45oC for 12 to 24 hours in air-sealed 250 mL teflon bottles, and filtered with a 1-mm polycarbonate filter in Ar(g). Treatments S1 and S2 (MR of 3.4) were studied to investigate the reproducibility of catalyst preparation. Treatment S3 (MR of 1.7) was prepared to examine whether increasing the amount of Hg influenced the catalyst form or activity. To determine whether high radiation had a negative influence on catalyst stability, treatment S4 was prepared under the same conditions of S1 and S2 but gamma-irradiated after equilibration at 45oC for 24 hours prior to filtration. Treatments SX, SY and SZ (MR’s of 3.4, 0.34 and 34, respectively) were prepared to examine the influence of Hg concentration on early cluster formation—after a 12-hour equilibration at 45oC.
Portions of the filtered solids were microwave-digested with aqua regia; Pd and Hg concentrations in the digests were determined by inductively-coupled mass spectrometry. The Pd and Hg speciation in the solids was analyzed using extended X-ray absorption fine-structure (EXAFS) and high-resolution transmission electron microscopy (HR-TEM) techniques (12).
Time-series measurements of dissolved Pd and TPB levels in the slurries indicate a loss of dissolved Pd concurrent with decomposition of TPB ion. Loss of Pd was most rapid in the treatments with low Pd:Hg starting ratios (data not shown; 4). These findings suggest some precipitation of Pd occurred during TPB decomposition. After 12-hours of equilibration, the mixtures experienced no change in color—they were light gray. Darker-colored solids formed in mixtures equilibrated for 24 hours or more—especially in the solids in the treatments with lowest MR of Pd/Hg. Quantification of the Hg concentrations in the solids indicates that 8 to 20% of the Hg initially added was in the solid phase (Table 1).
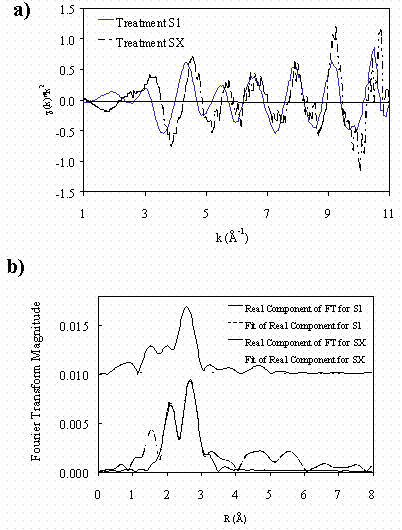
Information on the speciation of Pd and Hg in the samples, coordination number (N) and geometry, and near and next-nearest neighbor environment of the supported Pd and Hg were determined using EXAFS techniques. Representative chi data and Fourier-transformed EXAFS data for Pd and Hg with model fits for first-shell heavy atoms for Samples S1 and SX are shown in Figs. 1 and 2 (data for remaining samples not shown). Model fits of the Pd-EXAFS data confirm that the samples that were equilibrated for 24 hours contain face-centered cubic (FCC) Pd metal (Table 1). In contrast, FCC Pd(0) was not detectable by X-ray diffraction (XRD, data not shown). This observation may be explained if the sample Pd forms are small clusters of atoms with short-range structural order. Because FCC Pd metal in an infinite lattice has a coordination number (N) of 12, the smaller N (for first-shell Pd metal) obtained from the sample EXAFS analyses allowed for a cluster size estimation. Based on maximum site indices, FCC Pd clusters with an average metal N of 5 to 7 would have an average radius ~0.2 to 0.47 nm respectively and therefore be nanoclusters (13). Fits of the Pd-EXAFS data for treatments S1 through S4 show that on average each Pd in the cluster is coordinated to one or more light weight atoms (such as O, C or Na) with a bond length of ~0.2 nm (Table 1)—data not shown.
The Pd-EXAFS data analyses for treatments SX and SY were indicative of Pd-Pd and Pd-Hg first-shell interactions. The first-shell N values for Pd or Hg in treatments SX and SY did not exceed 12 (Table 1). Only Pd-Pd first-shell interactions were detected in treatment SZ (low Hg) and the N for the Pd was 6.7—suggesting Pd nanoclusters may have formed.
The Hg-EXAFS analyses indicate Pd as the only detectable metal in the first shell (of Hg) (Table 1; Fig. 2). The structure of the Hg was FCC and the Hg-Pd distances (from Hg-EXAFS) were equal to that of the Pd-Hg distances (from Pd-EXAFS)—suggesting the Hg and Pd share the a common coordination environment in the samples. For treatments S1 through S3, Hg had an N of 12. Treatment S4 had a coordination number of 9.76—indicating irradiation may influence Hg cluster size.
In contrast, the Hg-EXAFS spectra for treatments SX and SY had Pd and Hg in the first coordination shell. Analyses of the Hg-EXAFS data predicted first-shell Hg-Pd distances for treatments SX and SY were in good agreement with the Pd-Hg distances obtained from the Pd-EXAFS data analysis.
EXAFS cannot easily resolve two sub-shells if one shell is <10% of the other. Hence, we conclude that Hg-Hg bonding constitutes <10 mol% of the cluster in treatments S1 through S4. If all Hg is bound to Pd, which is present in the form of a nanocluster, Pd would be on the outside of the particles and Hg would be on the inside of the particles. This is because the value of the first shell N of Hg is nearly twice that of the first shell N for Pd. Moreover, if all Pd is in a nanocluster form and the N for Hg is 12, the HgPd cluster size would be constrained by the cluster size of the Pd.
Furthermore, EXAFS analyses give average information on atomic distances, coordination and cluster size but it cannot discern between two or more populations of clusters. The HR-TEM techniques complemented the interpretations of the EXAFS data. The HR-TEM analyses of treatment S1 revealed two populations of particles with Pd present as small 3 to 10-nm sized Hg-free nanoclusters and as coarser-grained 100-nm thick Hg-rich alloy film (Fig. 3a). Lattice fringe field emission TEM images indicate the Pd particles in treatment S1 were 3 to 10 nm in diameter (Fig. 3b), which further confirms that nanoclusters are present and stable in air (14). Selected area electron diffraction (SAED) studies indicate that the coordination environment of the metals is FCC, in agreement with the EXAFS data (SAED images not shown).
For catalytically reactive bimetallic nanoclusters, the increase in reactivity behavior has been attributed to charge transfer (ligand effects) and modification of multi-atom surface sites (ensemble effects) (15). The structural incorporation of atoms with a significantly larger or smaller radius than that of the host atoms may promote the formation highly defective solids, with numerous defects at grain interfaces, dislocations and phase boundaries within the crystallites that can serve as highly reactive surface sites (10). Typically, metallic Hg poisons zero-valent noble metal catalysts and to date, no studies demonstrate that metallic Hg plays a synergistic role in catalysis by zero-valent noble metals.
In summary, these studies demonstrate that catalysis occurs in the presence of Pd(0) and Hg(0) and bimetallic Hg-Pd nanoclusters in these reactive systems. Metallic Pd and Hg in bulk solids is observed (16) but few if any studies report the presence of catalytic nanocluster forms of noble metals and Hg(0). Whether Hg is reactive in this form remains to be determined and whether Hg facilitates the formation of more reactive forms of Pd than those formed in reactive Pd-containing HLW simulants in the absence of Hg is yet to be determined. However, it is clear that Hg has a pronounced and unexpected synergistic effect on the decomposition of TPB in the presence of Pd. The presence of Hg in the Pd structure may result in the formation of numerous surface defects that could provide highly reactive sites for TPB catalysis. More information is needed about the behavior of noble metals other than Pd in these HLW simulant systems and in the presence of potential supporting materials such as Al, Fe and Mn oxides, which are present at percent levels in the tank sludge.
The high reactivity of catalytic nanoclusters of noble metals in the HLW tanks could explain the unexpectedly high levels of benzene observed during treatment with TPB. In the absence of phenylborate ligands or polyoxoborate, which could stabilize nanoclusters that could form during HLW treatment with TPB ion, the high levels of Na+ ion in the tanks could stabilize any nanoclusters (if present) and prevent their coalescence into larger less reactive particles. Therefore, modestly low levels of noble metals, which occur as nuclear fission and decay products in the HLW could have considerable influence on TPB catalysis during treatment. These studies provide more information about the fundamental mechanisms of TPB decomposition. A better understanding of these complex systems will aid in the development of treatment strategies—should precipitation of radiocesium with TPB ion be considered in the future.

Figure 1. Palladium-EXAFS data for treatments
S1 and SX a) chi data and b) Fourier-transformed chi data.
The EXAFS data for treatments S1 through S4 were nearly identical. Data for
treatments
SX and SY (not shown). Data for treatment SZ was similar to that of treatments
SX and SY
but possessed a lower signal to noise (data not shown).
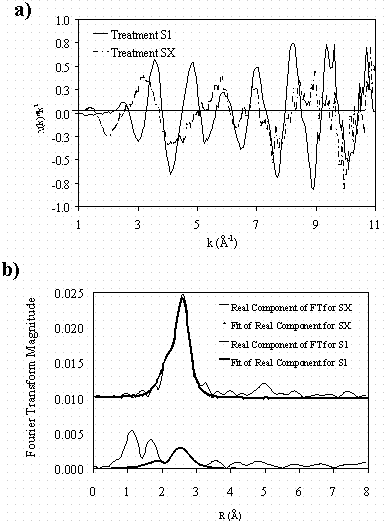
Figure 2. Mercury-EXAFS data for treatment
S1 and SX a) chi data and b) Fourier-transformed chi data.
The EXAFS data for treatments S1 through S4 were nearly identical. Data for
treatments
SX and SY (not shown) were similar. Data for treatment SZ possessed a very low
signal to noise and fits were not possible (data not shown).
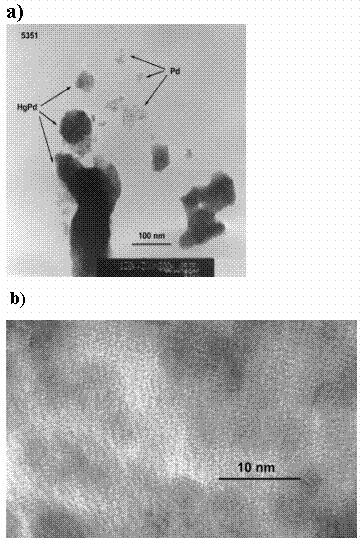
Figure 3. HR-TEM studies showing a) images
of small Pd nanoclusters and a PdHg alloy of 10-nm thickness
and b) lattice fringe measurements of 3- to 10-nm sized Pd nanoclusters.
Table 1. Description of sample treatments and first coordination shell fit results from the Pd- and Hg-EXAFS analyses.
|
Sample Treatment or |
Solid Pd:Hg Mole Ratio (MR) |
First-shell |
Distance |
N |
s 2[nm]2 |
|
S1 (initial Pd:Hg MR of |
9.82 ± 0.10 |
Pd-Pd Hg-Pd |
0.277 0.278 |
7.01 11.95 |
0.0000643 0.0000889 |
|
S2 (S1 replicate, initial |
10.38 ± 0.4 |
Pd-Pd Hg-Pd |
0.277 0.279 |
6.16 12.24 |
0.0000562 0.0000829 |
|
S3 (initial Pd:Hg MR of |
4.31 ± 0.01 |
Pd-Pd Hg-Pd |
0.277 0.279 |
5.08 11.97 |
0.0000689 0.0000817 |
|
S4 (initial Pd:Hg MR of 3.4* |
9.83 ± 0.08 |
Pd-Pd Hg-Pd |
0.277 0.280 |
5.55 9.76 |
0.0000733 0.0000813 |
|
SX (initial Pd:Hg MR of 3.4)† |
ND‡ |
Pd-Pd Pd-Hg Hg-Hg Hg-Pd |
0.276 0.275 0.277 0.274 |
4.66 6.30 4.94 5.21 |
0.0000269 0.0000873 0.0002538 0.0001399 |
|
SY (initial Pd:Hg MR of 0.34)† |
ND |
Pd-Pd Pd-Hg Hg-Hg Hg-Pd |
0.275 0.281 0.290 0.279 |
1.52 10.1 5.20 3.81 |
0.0000393 0.0001369 0.0001569 0.0000985 |
|
SZ (initial Pd:Hg MR of 34)† |
ND |
Pd-Pd Pd-Hg Hg-Hg Hg-Pd |
0.275 ND‡ ID§ ID |
6.70 ND ID ID |
0.0000393 ND ID ID |
|
Pd FCC metal EXAFS foil. |
NAï ï |
Pd-Pd |
0.276 |
12.02 |
0.00313 |
|
Hg(0) rhombohedral simulation. |
NA |
Hg-Hg |
0.301 |
6.00 |
NA |
References and Notes