WSRC-MS-2001-00248
J. E. Klein
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/index.html
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Bench scale methane cracking tests have been completed using a stack of ten whole SAESÒ St909 pellets -- a 6 gram sample size. Baseline test conditions were five vol% methane in helium at a flow of ten sccm, 101 kPa (760 torr), and 700°C. Variations from baseline conditions include tests at 650°C, 800°C, 50.7 kPa (380 torr), 203 kPa (1520 torr), 12.5 sccm, 15.0 sccm, 17.5 sccm, 20.0 sccm, and carrier gas binary mixtures of helium/hydrogen, helium/nitrogen, and hydrogen/nitrogen. Tests were also conducted with various binary mixtures of methane and helium, hydrogen, or nitrogen over the range of 2.5 vol% to 100 vol% methane at 10 sccm, 101 kPa, and 700°C.
Temperatures of 800°C gave the best methane cracking performance and efficiencies decreased rapidly with temperature. Increasing the gas feed flow rate decreased methane cracking efficiency, but tests run for the same duration had a higher weight gain at higher gas feed rates. Methane cracking efficiency was increased at a higher pressure and was reduced at a lower pressure, but was not a simple function of feed gas residence time.
Increasing the hydrogen and/or nitrogen composition of the carrier gas, or the introduction of ammonia into helium, reduced the methane cracking efficiency. Ammonia was cracked by St909 at high efficiencies, but greatly reduced the methane cracking efficiency. Hydrogen in the carrier gas or from methane cracking was thought to reduce net methane cracking efficiency by reforming with carbon present on the surface of the St909. Nitrogen reacts with St909 to inhibit methane cracking. The combination of nitrogen and hydrogen in the carrier gas reduces methane cracking efficiencies further than using a pure nitrogen or hydrogen carrier gas.
Further studies of the methane cracking data are needed to understand the minima-maxima results seen from tests run at 700°C. Continued bench scale testing and larger scale tests are needed to better understand St909 methane cracking performance in a full scale St909 bed.
The Savannah River Site (SRS) Tritium Facilities have various process gas streams that include impurities such as water, carbon monoxide, carbon dioxide, methane, and ammonia. Removal of these tritiated impurities is needed to minimize emissions for the tritium processing facilities and removal of carbon oxides is needed to prevent poisoning or comsumption of process beds.
Tritium cleanup using a series of heated uranium beds has been proposed,1 but carbon dioxide in a hydrogen carrier stream was found to produce methane under certain operating conditions.2 Pd-Ag alloy diffusers, or permeators, are routinely used to purify and process tritium,3 but the effect of impurities on the long-term performance of the diffusers is still being studied.4
New SRS tritium facility projects will process gas stream impurities using a SAESÒ St909 bed before final hydrogen isotope removal by diffusers. St909 is a Zr-Mn-Fe alloy getter material developed to dissociate, or "crack", tritiated water for tritium recovery.5 The alloy retains relatively low amounts of hydrogen6, and has been demonstrated to remove CO and CO2 from inert streams7as well as remove O2, and crack NH3 and methane.8,9 St909 is 40.5 wt% Zr, 24.5 wt% Mn, 25 wt% Fe, and 10 wt% Al. The supplied material is advertised as a single-phase ZrMnFe alloy with the aluminum used as a binder for pellet formation.
Methane was found to be the most difficult impurity to process2 and can impact diffuser operation.4 The most systematic study of methane cracking using St909 used 0.1% and 1.0% methane in a helium carrier gas was studied over a range of operating conditions.9 In this work, bench scale methane cracking tests were performed to examine methane cracking performance at higher methane concentrations, at different pressures, at different flow rates, with different carriers gases, and at a the proposed SRS St909 vessel design temperature of 700°C. The use of ten whole pellets for bench scale testing did not allow prediction of full-scale reactor methane cracking efficiencies, but did allow the study of how different operating conditions impacted methane cracking efficiency.
Methane cracking tests were divided into Test Sets (TSs). Baseline conditions for comparison of other test results were five vol% methane in helium at a flow of ten sccm, 101 kPa (760 torr), and 700°C. TS conditions varied from baseline conditions and are summarized in Table 1. For example, TS 7, TS 10, and TS 11 varied the methane composition in a single carrier gas from 2.5 vol% to 100 vol% methane at 10 sccm, 101 kPa, and 700°C.
Table 1. Test Set Description Summary
|
Test Set |
Variation from Baseline Conditions |
|
3 |
650 and 800°C |
|
4 |
12.5, 15, 17.5, and 20 sccm |
|
6 |
50.7 and 203 kPa (380 and 1520 torr) |
|
5 |
Carrier gas: helium plus hydrogen |
|
8 |
Carrier gas: helium plus nitrogen |
|
9 |
Carrier gas: nitrogen plus hydrogen |
|
7 |
Vary methane/helium ratio |
|
10 |
Vary methane/hydrogen ratio |
|
11 |
Vary methane/nitrogen ratio |
The St909 material was identified by SAESÒ Getters of Milan, Italy, as St909/PIECES/64. The pellets were cylindrical with an O.D. of 6 mm, a height of 4 mm, weighed approximately 0.6 grams , and had a metallic, silvery appearance. Ten fresh/unreacted, whole pellets were used for each test, approximately six grams per test, with each pellet uniquely identified using an engraver. Triplicate micrometer pellet diameter measurements were made before and after a test: fresh pellets consistently gave an O.D. of 6.07 mm (0.239 inches). Before and after each test, the pellets were weighed individually and the ten pellets weighed cumulatively to crosscheck individual weighings. A pellet’s stacking order in the test bed was also tracked. Helium, hydrogen, and nitrogen were used along with a 1.00 vol% argon/balance methane mix, and a 1.99 vol% anhydrous ammonia/balance helium gas mixture. Argon from a liquid argon boil-off tank was used for system flow testing.
The test reactor/bed was made from 9.53 mm (3/8 inch) O.D., 0.889 mm (0.035 inch) wall thickness, bright-annealed 316L stainless steel (SS) tubing. The pellets were held in place by a SS filter cup compressed ("Swaged") inside the tubing. At room temperature, the pellets would occupy 61 percent of the cross-sectional area of the reactor tube, gave an annular void volume of 0.728 cc for ten pellets, and a bed residence time of 1.2 seconds at baseline test conditions. Before each test, the empty test bed and the pellets were weighed before filling the bed and the filled bed also weighed to crosscheck measurements. The bed was mounted in a vertical orientation with gas feeding into the top and bed reaction products exiting out the bottom. After a test, the filled bed was weighed, the pellets removed, and the empty bed and pellets weighed separately.
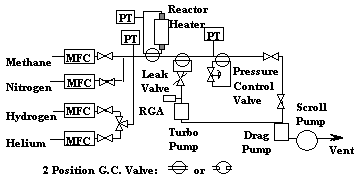
A simplified test system schematic is shown in Figure 1. 3.18 mm (1/8 inch) O.D. by 2.16 mm (0.085 inch) I.D. 316 SS tubing (1.12 cc per foot of tubing) was used along with gas chromatographic (G.C.) style valves to reduce dead volume. The G.C. valves functioned like two, three-way ball valves in their ability to valve-in or bypass a piece of equipment.

Figure 1. Simplified Test System Schematic
Gas flows were controlled using thermal bypass mass flow controllers (MFCs) calibrated using volumetric glassware measuring volume displacement with time and temperature and pressure corrected to standard conditions of 101 kPa (760 torr) and 0°C. Gas mixture compositions were calculated from MFC set point flow rates. Methane compositions are presented as "pure" methane values with the actual values reduced by one percent due to the use of the methane/ argon mix. Pressures were measured with pressure transducers (PTs) at the inlet to the bed, the outlet of the bed, and for leak-checking test bed connections. A pressure control valve located after the test bed was used to control the test pressure and exhausted to a molecular drag pump backed by a scroll pump which discharged to the hood exhaust.
A Leybold Inficon model TSPTT100 residual gas analyzer (RGA), atomic mass unit range up to mass 100, was used for testing. A Granville-Phillips Series 203 variable leak valve was modified to leak gas to the RGA and improve the response time of the RGA. Flow tests were performed by filling an empty test bed at ambient temperature with helium at 101 kPa (760 torr) and isolating the bed using the G.C. valve. A ten sccm argon flow at 101 kPa (760 torr) was established in the system and the flow switched from bypassing to flowing through the test bed. The lag time before seeing the first RGA helium signal and complete decay to its initial baseline value was virtually complete in seven minutes.
The argon in the methane was used as a tracer gas so methane cracking efficiencies could be calculated on a relative, rather than an absolute, basis. RGA data were collected of the feed gas by bypassing the test bed for ten to twenty minutes before and after the test with the test bed effluent being monitored continuously during a test. The ratio of the methane-to-argon signals, massCH4/massAr, were used to calculate methane cracking efficiency:
Efficiency = 1 – (massCH4/massAr)test / (massCH4/massAr)feed
where the subscripts "feed" and "test" indicate the feed gas ratio and the reactor effluent during testing ratio, respectively: the methane mass 16 signal and the argon mass 40 signal was used. The methane-to-argon ratio of the feed mixture before and after testing was averaged to calculate methane cracking efficiencies.
A ceramic fiber heater connected to a temperature controller supplied bed heating with the stack of ten St909 pellets in the center of the heated zone. An Inconel sleeve with two type K thermocouple (TC) troughs on one side and a trough machined on the other side to match the O.D. of the test bed was used to improve thermal contact between the test bed and the TCs: the TCs where held to the bed by wire ties.
After installing a test bed into the system, insulation was placed around the top and bottom of the heater to reduce heat losses from the bed and the bed evacuated to circa 0.133 to 1.33 Pa (1x10-3 to 1x10-2 torr) and rate-of-rise leak checks performed. Activation of the material was done by heating the bed to 600°C for two hours under a ten sccm flow of helium at 101 kPa (760 torr). It should be noted that the activation temperature was below the 660°C melting temperature of the aluminum used as a pellet binder material.
After activation, the test bed temperature and pressure were adjusted as needed to test conditions and the bed, filled with the activation gas at the test temperature and pressure, valved out of the flow path. After the feed gas flows were established, the leak valve adjusted to obtain the target RGA sensor pressure. A test was initiated by valving the test bed into the feed gas flow path via the G.C. valve. The test duration was chosen to be 27 hours for a nominal five vol% methane feed which would pass circa 800 scc of methane to the test bed. After collecting RGA data of the feed gas after the test, the system lines were flushed with helium, the helium flow set to 10 sccm and 101 kPa (760 torr) and the bed purged for nine minutes before isolation, cool-down, removal, and sample analyses.
Results
Methane cracking efficiencies were plotted as a function of standard cm3 (scc) of methane fed to the test bed, instead of time, so efficiencies at different flow rates and methane concentrations could be compared on the same basis. Figure 2 shows the effect of temperature for TS 3, a repeated baseline test, and the effect of pressure for TS 6. Figure 3 shows the effect of gas flow rate for TS 4.
Figure 2 shows that methane cracking efficiency is a strong function of temperature and was significantly increased when the pressure was doubled, but was not reduced by the same magnitude when the pressure was reduced by a factor of two. Figure 3 shows, as anticipated, methane cracking efficiencies are higher at lower flow rates, i.e. longer residence times, and decreased as flow rate increased.

Figure 2. Test Set 3 and 6 Methane Cracking Efficiencies
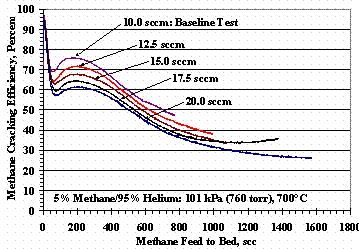
Figure 3. Test Set 4 Methane Cracking Efficiencies
Figures 4, 5, and 6 show the effects of varying the carrier gas compositions from the 95 vol% helium baseline compositions. Figure 4 shows TS 5 where the carrier varied from 95 vol% helium to 95 vol% hydrogen, Figure 5 shows TS 8 where the carrier varied from 95 vol% helium to 95 vol% nitrogen, and Figure 6 shows TS 9 where the carrier varied from 95 vol% hydrogen to 95 vol% nitrogen. Figure 4 shows as the hydrogen concentration increased, the methane cracking efficiency decreased and was reduced to where the minima-maxima (min-max) in the data disappeared. Figure 5 shows the introduction of 5 to 15 vol% nitrogen in the carrier gas drastically altered the shape of cracking efficiency plot while higher concentrations of nitrogen gave essentially the same, reduced efficiency results. Figure 6 shows the methane cracking efficiencies were smaller when both nitrogen and hydrogen were present than when the carrier gas was nitrogen or hydrogen alone.
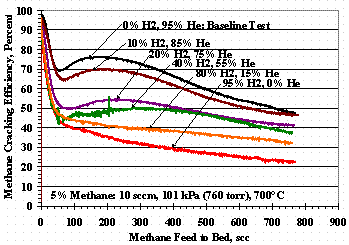
Figure 4. Test Set 5 Methane Cracking Efficiencies
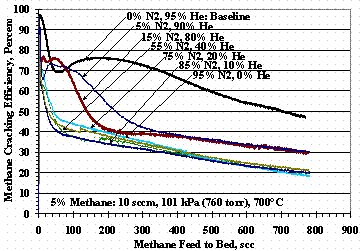
Figure 5. Test Set 8 Methane Cracking Efficiencies
Figures 7, 8, and 9 show methane cracking efficiencies as the ratio of methane-to-carrier gas composition varies. Figure 7 shows TS 7 where the methane in helium varied from 2.5 vol% to 100 vol%, Figure 8 shows TS 10 where the methane in hydrogen varied from 2.5 vol% to 100 vol%, and Figure 9 shows TS 11 where the methane in nitrogen varied from 2.5 vol% to 100 vol%. Figure 7 shows the cracking efficiency was higher at lower methane concentrations, but as methane concentration increased, the min-max shape disappeared. It is interesting to note the methane cracking efficiency was greater for 100% methane feed than for 80 to 90 vol % methane feed.
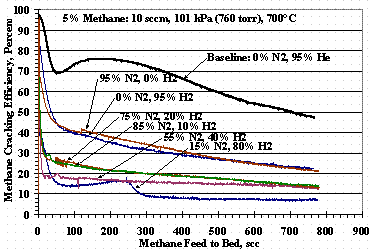
Figure 6. Test Set 9 Methane Cracking Efficiencies
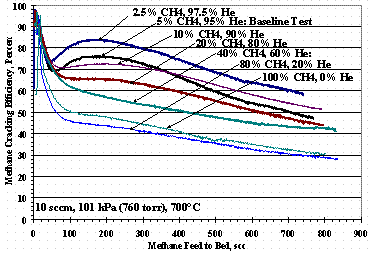
Figure 7. Test Set 7 Methane Cracking Efficiencies
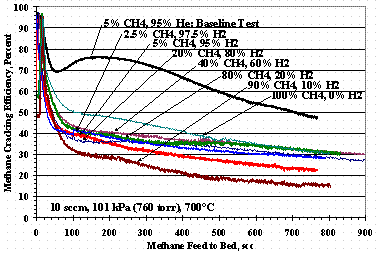
Figure 8. Test Set 10 Methane Cracking Efficiencies
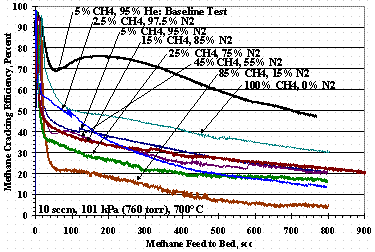
Figure 9. Test Set 11 Methane Cracking Efficiencies
The post-test pellets appeared darkened black compared to pre-test pellets. The pellets remained intact with insignificant fines created. No loose soot was apparent on the pellets or as a result of handling the pellets.
Figure 10 shows the weight change for the individual pellets as a function of test bed position for TS 3: pellet 1 indicates the bottom of the pellet stack and pellet 10 the top pellet of the stack. Figure 10 shows that at 800°C, a significant weight change gradient was present from the inlet to the outlet of the reactor. The parabolic-like pellet weight change profile shown in the figure at 650°C was prototypic of many tests with profiles shifted up or down for higher or lower weight changes. Some tests had profiles like those obtained at 700°C while others showed the opposite trend -- the outlet pellet weight change greater than the inlet pellet weight change.
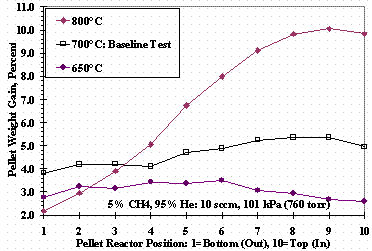
Figure 10. Test Set 3 Pellet Weight Changes
Figure 11 shows the weight change of St909 pellets, relative to their initial mass, as a function of the binary carrier gas composition for TS 5, TS 8, and TS 9. Figure 11 shows that for TS 5, increasing the hydrogen concentration of the hydrogen/helium carrier gas resulted in a decreased weight change. Conversely for TS 8, increasing the nitrogen in the nitrogen/helium carrier gas increased the weight change although, Figure 4 and Figure 5 showed similar methane cracking efficiencies at 95 vol% hydrogen carrier gas and 95 vol% nitrogen carrier gas, respectively. TS 4 data would show a nominal 0.1 percent increase in weight gain per sccm increase in flow rate.
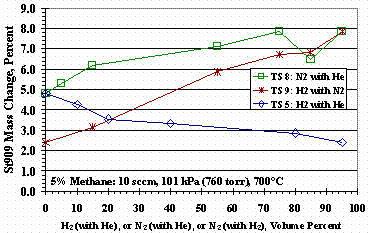
Figure 11. Test Set 5, 8, and 9 Bed Weight Changes
Figure 12 shows the weight change of St909 pellets as a function of the pure carrier gas composition for TS 7, TS 10, and TS 11: the data for TS 10 at 60 % and 80 % hydrogen had more methane exposure than the other tests and were left off Figure 12. The TS 10 data in Figure 12 show the pellets gaining roughly the same mass over the entire range of methane compositions up to 5 % methane where it drops about one percent for a 95 % hydrogen carrier. For TS 7 there was a modest increase in mass gain as the methane concentration in the helium carrier gas decreased. Conversely for TS 11, increasing the nitrogen in the methane-nitrogen feed increased the mass change although, Figure 9 shows poorer cracking performance than TS 7 data shown in Figure 7.
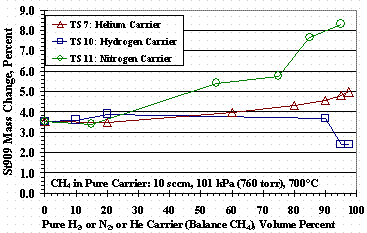
Figure 12. Test Set 7, 10, and 11 Bed Weight Changes
To determine if incorporating carbon into St909 caused pellet swelling, tests were conducted without pellet exposure to methane at a gas flow of ten sccm gas flow at 101 kPa (760 torr). Sample #13A was three pellets heated for two hours in helium at 600°C, as was done during pellet activation; Sample #14A was three pellets heated for two hours in helium at 800°C; and Sample #13C was three pellets heated for 141 hours in helium at 600°C. Sample #13B was three pellets heated for two hours in helium at 600°C followed by two hours of hydrogen flow at 600°C while Sample #14C was three pellets heated for two hours in helium at 800°C followed by two hours of hydrogen flow at 800°C. After the two hours of hydrogen the flow, the bed was isolated in the hydrogen atmosphere and allowed to cool in an attempt to make a metal hydride and swell the pellets. Sample #14D was ten pellets heated for 259 hours in helium at 700°C.
Figure 13 shows the change in pellet diameter as a function of weight change for individual pellets, with and without methane exposure. The region of near-zero weight change shows pellet diameter changes without methane exposure just from heating the pellets. Most pellets exposed to methane had slightly larger diameter changes than those without methane exposure. For some methane cracking tests, a trend of increased diameter change with increased mass change could be found, but this trend could not be generalized to all the data. Anomalous results for TS 3 at 800°C showed various diameter changes at circa 10% weight change and virtually the same diameter change for a range of weight changes instead of the clustering of data points found for the other tests.
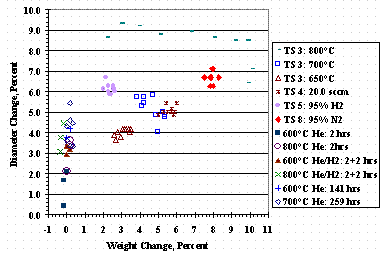
Figure 13. Pellet Swelling Versus Weight Change
Discussion
These bench scale tests, due to the use of whole pellets and the scale of the test system, can not be used to predict performance of full scale reactors, but were useful in systematically measuring the effect of different experimental parameters on methane cracking efficiency of the St909. These results could be used as lower bound estimates of methane cracking efficiencies of a full-scale reactor.
The reason for the min-max, the decrease, increase, and then decrease in the methane cracking efficiency plots at 700°C, is not clear. The seven minutes determined to displace the test beds’ activation gas at 10 sccm corresponds to 3.5 scc methane fed to the bed for a 5 vol% methane feed while the location of the minima of the methane cracking plots in Figures 2, 3, and 4 occured at higher values in the range of 50 to 90 scc methane. For TS 3, the min-max did not occur at 800°C because there was no sharp decrease in initial efficiency. It appeared at 700°C, and then disappeared at 650°C because the initial drop in efficiency did not increase. For TS 6 at 700°C and 203 kPa (1520 torr), the min-max almost disappeared, but was still present at 50.7 kPa (380 torr).
Figure 10 at 800°C shows the pellet weight change profile expected if the methane cracking rate was sufficiently high: the inlet of the test bed was exposed to the highest concentration of methane, cracks methane reducing its concentration, leaving less methane available for cracking and weight gain for pellets near the exit of the bed. The pellet profiles at other temperatures are more characteristic of a mixed-tank reactor: uniform in their net cracking performance based on weight change. The parabolic shape of the pellet weight change profiles was attributed to temperature variations along the length of the test bed from the single zone heater. A profile with a modest slope was attributed to temperature gradients caused by insufficient installation of the insulation at the top or bottom of the test bed.
The effect of residence time and pressure can be contrasted by examining the results in Figure 3 and for TS 6 in Figure 2. The Figure 3 minima occur at roughly 56 scc methane fed to the bed at 10 sccm and increase by approximately 1.8 scc methane fed per sccm total feed rate: a range of 56 to 75 scc methane feed. The TS 6, 50.7 kPa (380 torr) test had the same 0.61 second residence time and approximate location of its minimum cracking efficiency as the TS 4, 20 sccm test, but the efficiency increased to that of the 12.5 sccm test at circa 225 scc methane and had similar performance to the 12.5 sccm test for its duration. The TS 6, 203 kPa (1520 torr) test had a residence time of 2.5 seconds, twice the residence time of the baseline test, and had better cracking performance than the baseline test.
Figure 4 shows methane cracking efficiency decreased as the concentration of hydrogen in the carrier gas increased. It is conjectured that carbon on the surface of the St909 from methane cracking reforms with hydrogen in the carrier gas giving a net reduction in cracking efficiency. This theory is supported by the hydrogen/helium data in Figure 11 showing a decrease in total weight gain, i.e. inhibiting incorporation of carbon into the St909, as the hydrogen concentration in the carrier gas increases. The TS 7 data in Figure 12 was interpreted as a decrease in the amount of methane in the feed, and thus the amount of hydrogen produced during cracking, allowed greater carbon incorporation into the St909 without methane reformation with the hydrogen. Figure 12 TS 10 data show methane reformation was likely whether the hydrogen was supplied in the carrier gas or was from the methane cracking reaction.
Figure 5 shows methane cracking efficiency decreased as the concentration of nitrogen in the carrier gas increased, but for a reason different than using hydrogen with helium. Figure 11 shows that as the nitrogen concentration in the carrier gas increased, the mass gain of the pellets increased even though the methane cracking efficiency for 95 vol% nitrogen carrier gas (Figure 5) gave almost identical results to those for a 95 vol% hydrogen carrier gas (Figure 4). TS 11 data in Figure 12 also shows that as the nitrogen concentration in the methane-nitrogen mixture increased, the mass change increased even thought TS 11 cracking efficiencies were similar to those for TS 10. The mass gain with nitrogen was interpreted as nitrogen absorbing or reacting with the St909 and reducing the ability of St909 to crack methane. Figure 6 shows the effect of nitrogen and hydrogen on methane cracking is additive: the nitrogen reduces methane cracking and hydrogen reforms with carbon from previously cracked methane to further reduce net cracking efficiency.
To examine the competition between methane cracking and nitrogen absorption via ammonia cracking, a 2 vol% ammonia test was done where the baseline carrier gas was replaced with a 93 vol% helium and 2 vol% ammonia carrier gas. Methane and ammonia cracking efficiencies were calculated relative to the mass 40 signal using the methane mass 13 fragmentation signal and the mass 17 signal, respectively. The methane and ammonia cracking results are shown in Figure 14 along with methane cracking efficiencies with 95% hydrogen and 95% nitrogen carrier gases.
Figure 14 shows the cracking efficiency of the 2% ammonia test was high, even in the presence of 5 % methane, and was 97% at the end of the test: the mass change of the pellets was 4.5 and was only 0.3% lower than the value shown in Figure 11. A test run for the same duration with the 2% ammonia in helium mixture (without methane) yielded an ammonia cracking efficiency of 99.9 % for the majority of the test and a pellet mass change of only 1.2%.
The plot in Figure 14 for the 2% ammonia test shows the initial methane cracking efficiency was greatly reduced, recovered to within 7 % of the efficiency for the baseline test, and then decreased to the efficiency of the nitrogen carrier gas test. Figure 15 shows selected RGA ratio data for the 2% ammonia test. As shown in other ammonia cracking work,9 there was a sharp initial decrease in the ammonia signal, a lag time, and then a rise in the nitrogen signal (28/40 ratio) although, it is not clear if the test was run longer if a constant nitrogen outlet signal would have been obtained. Results for feeding 2% ammonia without methane gave (28/40) results similar to those shown in Figure 15 and would also show a (2/4) plot which was quickly established and constant during the test as was found before.9 These data further support the absorption or reaction of nitrogen with the St909.
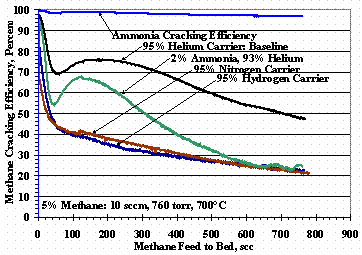
Figure 14. Methane Cracking Efficiency with Ammonia
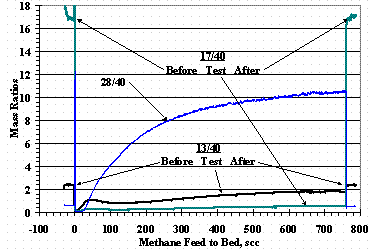
Figure 15. 2% Ammonia RGA Mass Ratio Data
Conclusions
St909 can crack methane under a variety of operation conditions, but the efficiency can be affected by many factors. The methane cracking efficiency was not as high as found in other work with similar residence times9 due to the use of a stack of whole pellets for the tests, which proportionately had a much smaller active surface area per volume for reaction, and higher methane concentrations.
Temperature had the greatest impact on methane cracking efficiency as St909 carbon content increased. An anomalous decrease, then increase followed by decrease in methane cracking efficiencies were found for many tests at 700°C and the reason for this behavior requires further investigation. Increasing test pressure increased methane cracking efficiency, but reducing the pressure did not decrease methane cracking efficiency by the same proportion. Increased flow rates gave reduced methane cracking efficiencies, but higher total weight changes for tests run for the same duration.
Changing the carrier gas composition from helium to hydrogen, nitrogen, or binary gas mixtures of these gases showed a decrease in methane cracking efficiency. When using a nitrogen or hydrogen carrier gas, Figure 14 showed that net methane cracking efficiency was almost the same, but Figure 12 showed drastically different weight gain results. These data lead to the conclusion that hydrogen inhibited the net methane cracking efficiency by reforming with carbon on the St909 surface while nitrogen reacted with the St909 reducing its effectiveness for methane cracking: nitrogen and hydrogen in the carrier gas combine both mechanisms further reducing methane cracking efficiency. Tests have been proposed using methane in a deuterium carrier gas or using deuteromethane in a hydrogen carrier gas stream to test the methane reformation hypothesis by looking for deuterium or hydrogen substituted methanes.
Pellet diameter changes can occur from heating the pellets and does not require the addition of carbon, hydrogen, or nitrogen. Solid state phase changes or the softening of the pellet aluminum binder may account for pellet diameter changes. For pellets without methane exposure, time at temperature appears to determine the extent of diameter change. Only the TS 3 test at 800°C produced anomalous results, i.e. a variation of diameter changes in the range of circa 6.5 to 9.5 percent for a variety of mass changes.
Larger, pilot scale, tests are planned to aid in predicting full-scale reactor performance. Further bench tests will examine the influence of oxygen containing species, i.e. carbon dioxide, to determine its impact on methane cracking efficiency.
Acknowledgments
The author would like to thank Bob Hsu and Jody Dye for their contributions to this work. This document was prepared in connection with work done under Contract No. DE-AC09-96SR18500 with the U.S. Department of Energy.
References