WSRC-MS-2000-00197
Microwave Remediation of Emissions Resulting from Treatment of Electronic Components
R. L. Schulz and G. G. Wicks
Westinghouse Savannah River Company
Aiken, SC 29808
D. E. Clark
University of Florida
Gainesville, FL 32611
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Abstract
The global community has become increasingly dependent on computer and other electronic technologies. As a result, society is faced with an increasing amount of obsolete equipment that is usually disposed of in landfills. While a convenient solution, this action causes a substantial loss of finite resources and poses a potential environmental threat as the various components breakdown and are exposed to the elements. Hazardous compounds such as lead, mercury, and cadmium may leach from the boards and find their way into the groundwater supply. In order to alleviate this potential problem, a microwave waste treatment system was developed that was capable of removing the organic compounds from the circuitry. Upon further heating in an industrial microwave, a glass and metal ingot were recovered. Analysis of the ingot revealed small concentrations of precious metals such as gold and silver. During treatment, gaseous organic and aromatic compounds were generated in the initial stages of processing. These emissions were successfully treated in a microwave off-gas system that reduced the concentration of the products emitted by several orders of magnitude and in some cases, completely destroyed components within the waste gas. In order to better understand the effects of processing parameters on the efficiency of the off-gas system, a parametric study was developed and undertaken. The study tested the microwave system at 3 incoming flow rates (10,30, and 50 ft3/min) and 3 temperatures (400, 700, and 1000°C). In order to determine the effects of microwave energy, some of the experiments were repeated using a conventional furnace in place of the microwave off-gas unit.
Introduction
Microwave treatment of electronic circuitry has been discussed in previous publications. In summary, on a laboratory scale, microwave energy proved to be effective in reducing to ash a wide variety of circuitry, vitrifying the residue ash, recovering metallic components, and simultaneously reducing the concentration of gaseous effluents. This paper provides information on the results obtained from treating emissions from circuit boards as well as single gas experiments, using microwave energy as well as a conventional heat source. Other process parameters that were varied during the study included flow rate of the air used to move the emissions to the off-gas chamber and the temperature of the off-gas chamber.
Experimental
Single Gas - Toluene Experiments
To better understand the effects of flow rate and temperature within the microwave off-gas system, it was decided that introducing a controlled amount of a single material would be beneficial. It was believed that this initiative would provide a known starting point for all experiments with reductions in concentration after microwave treatment due solely to changes in processing parameters, rather than variations in initial feed material or fluctuations in the volume of the waste gas species. However, the data collected could not be considered quantitative as a mass balance could not be calculated without knowing the amounts of carbon dioxide, water vapor and other decomposition products generated. Toluene was selected for use in the study because it was generally found in the waste emissions generated during processing of electronic circuitry, was readily available, and had fewer health risks associated with its use when compared to some of the other gases considered (benzene).
For each experiment, the air flow rate was adjusted to either 10, 30 or 50ft3/min. Temperature of the off gas unit was pre-set to 400, 700, or 1000°C and the Tenax air traps were positioned in the sampling ports. Liquid toluene was then introduced to the compressed air line through a septum using a gas-tight glass syringe. The septum was positioned approximately 5 feet from the lower microwave chamber air inlet opening in order to allow adequate mixing and vaporization of the toluene before it reached the pre-microwave treatment gas sampling port and the off-gas chamber (either microwave or conventional heating). The toluene was injected incrementally (100µl/minute ) until the total amount was delivered to the air stream (1000µl).
Pantex Defense Electronic Circuitry Experiments
To begin this study a specific board type was selected. These boards were crushed and combined together. Then, in order help ensure a somewhat uniform feed, the circuit board laminated material was separated from the crushed chips and other surface mount material that detached from the boards during the crushing operation. For each experiment, approximately 25 grams of laminate material and 9.5 grams of surface mount material was used. Processing time was 15 minutes. The sample material was placed in a fused silica crucible. The crucible was situated within the Rath refractory cylinder and a K-type Inconel-shielded thermocouple was positioned in the sample. A Kaowool board cover with a circular opening was placed on top of the setup and the entire assembly was positioned beneath the opening of the off-gas interconnect. An air flow rate was selected and the Tenax air tubes were positioned. The original experimental plan consisted of a series of 18 experiments and each was to be repeated 2 times. However, after reviewing the results from the toluene study and data from the microwave off-gas tests, a number of tests were eliminated from the conventional heating portion of the experiment.
The conventional off-gas unit consisted of a Thermolyne tube furnace lined with an alumina tube. In order to simulate conditions that existed in the microwave off-gas unit, SiC filters and phosphate-bonded filters were positioned within the tube to provide the same bed length as was found in the microwave system.
Results
Single Gas Experiments - Toluene Study
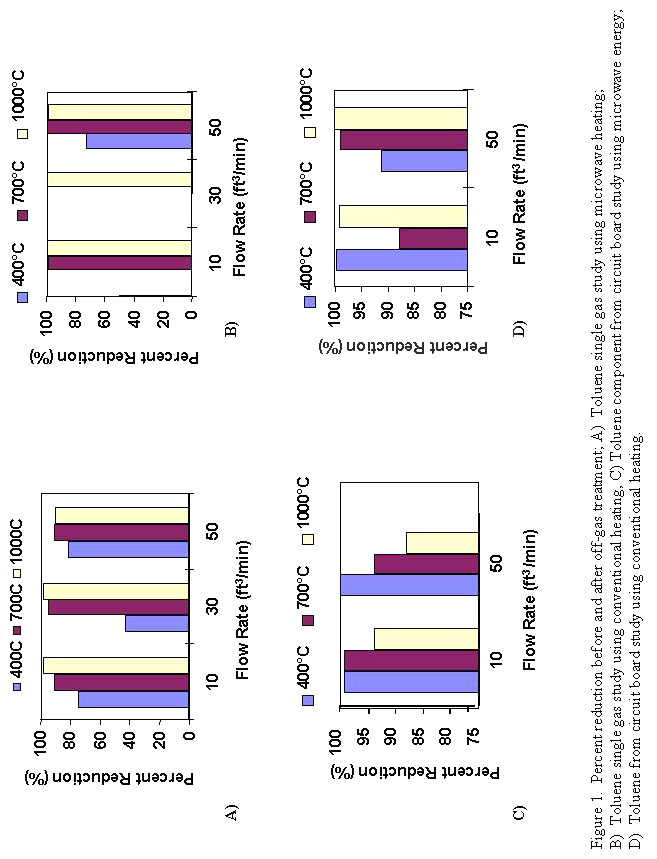
The results of the toluene study are summarized in Figure 1. As can be seen from the data, there is little difference in the performance of the microwave system when compared to the conventionally heated system. It did appear that both systems performed somewhat better at higher temperatures (700-1000°C). This is to be expected as the autoignition temperature for toluene is 553°C. Autoignition temperature is defined as the temperature where the gas will combust. To sustain combustion, an excess of air is required and the concentration of gas to be combusted must be maintained between the lower and upper explosion limit (1.3-7.0 % by volume for toluene)10. The decomposition reaction is as follows:
C7H8 + 9O2 è
7CO2 + 4H2O
553°C
Since this exact ratio was most likely not attained with the current system, it is reasonable to assume that other combustion by-products were present (for example, CO) and complete destruction of toluene was not attained. This supposition is supported by the data in cases where the percent reduction was less than 100%. The data also show that for the flow rates selected, there was no real discernable difference with changes in flow rate.
There also did not appear to be a real difference between heating methods. There are several possible reasons for this. The first, is that microwave energy, at this frequency, has no effect on toluene. A second possibility may be that the SiC used to attain the temperatures necessary to decompose the toluene absorbed most or all of the microwave energy and the only heating taking place within the off-gas chamber was through radiant heating.
For the systems tested in this study, residence time was a factor of flow rate. In the microwave off-gas system, the refractory cylinder was packed with SiC reticulated ceramic filters and phosphate-bonded alumina filters. Each filter had a volume of approximately 1.9 x10-4 ft3 and there were a total of 9 filters.

The cylinder had a volume of 1.62 x 10-2 ft3. To calculate the residence time as a function of flow rate, the following equation was used:


By integrating from t = 0 to t = t, assuming a first order reaction(n = 1), inserting known values for k (at 760°C), cA (0.001 for a 99.9 removal efficiency) a residence time equal to approximately 0.2 seconds is calculated for benzene. Similar calculations carried out for a temperature of 538°C yields a residence time of 17.4 hours. Therefore, the dependence on temperature is clearly seen. It should also be noted that the microwave system was able to reduce the concentration of benzene by 99.9% at 400°C and a flow rate of 10 ft3/min. This corresponds to an estimated residence time of 0.087 seconds. Similar results were obtained for flowrates of 30 and 50 ft3/min, corresponding to even shorter residence times.
As can be seen in the GCMS data tables, a percent difference column was included. This value was calculated using the following formula:
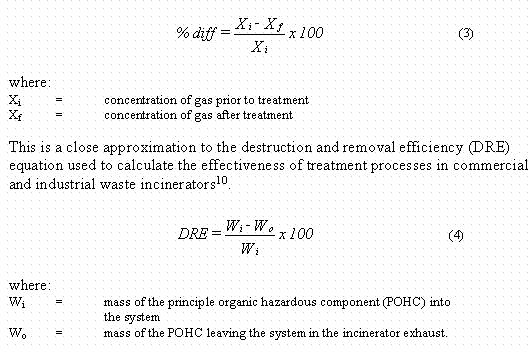
For most hazardous wastes, a DRE of at least 99.99% is required in order for the incineration process to be considered effective. For some species, a DRE of 99.9999% is required (dioxin, polychlorinated biphenyls, furans). Since the mass of the aromatic hydrocarbons entering and leaving the off-gas was unknown, a calculation of the percent difference in concentration of the species detected in the air trap by GCMS provides only an approximation of the efficiency of the microwave and conventional systems tested during this study.
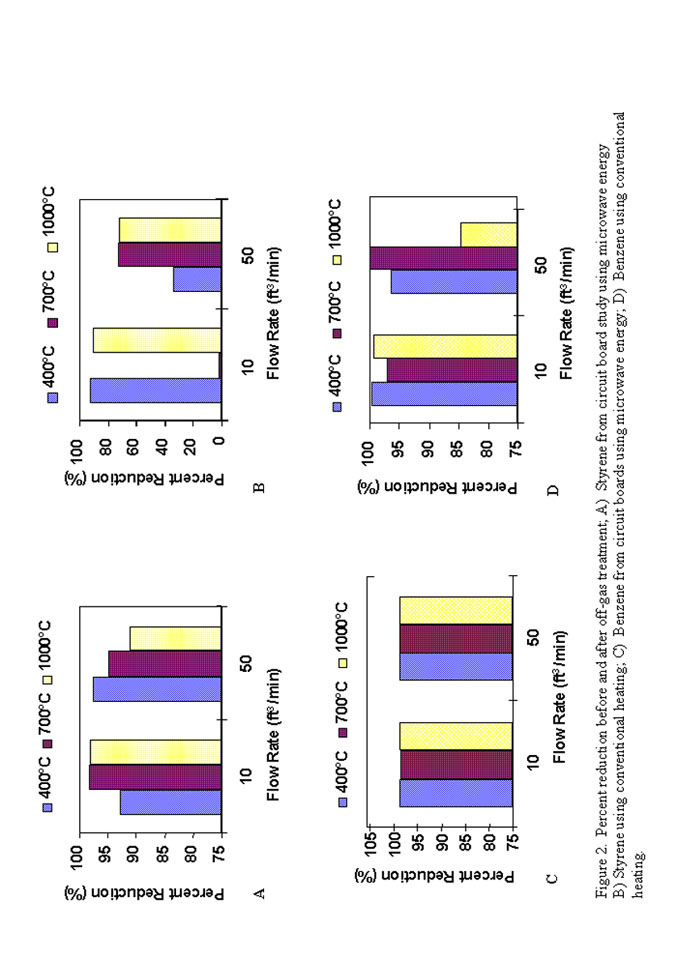
The gas chromatography-mass spectroscopy data collected for the microwave portion of the study is summarized in Figures 1(C,D) and 2 which show the percent reduction in toluene, styrene and benzene as a function of temperature, flow rate and heating method. The GCMS data generated from the Pantex circuit board study is consistent with the single gas toluene data in that there was no clear distinction between microwave and conventional heating, regardless of the organic compound, temperature or flowrate. The only species where one may make a case for a difference between heating methods is styrene, where it appears that microwave energy may be slightly more efficient in decomposing this compound. However, further testing is necessary to be sure the results consistently repeat this trend.
In some cases, gas compounds were found in the air stream that were either, not present, or found in smaller concentrations in the pre-treated sample
(bromobenzene and 1,2,4 trimethylbenzene). One proposed explanation may be that these compounds represent intermediate breakdown products of other species in the waste gas and that the temperatures or residence times in the off gas system were insufficient to destroy them prior to release. However, if this were the case, one would expect this trend to be found consistently throughout the study. This was not the case. Therefore, this phenomenon remains unexplained and warrants further study.
Overall, the results of this study reveal that both systems appear to function well and are reasonably efficient in destroying a mixed waste gas air stream consisting primarily of aromatic hydrocarbons. As these compounds represent a threat to the health of the population given sufficient exposure and concentration, the value of the microwave waste gas system is clear. However, to develop the microwave system to its full potential requires additional study.
Summary
A microwave waste treatment system was developed and was able to successfully treat all circuitry presented for processing thus far. Following vitrification, recovery of metals (including gold and silver) is possible offering a potential return on investment. The microwave off-gas system was successful in decreasing the concentration of a wide variety of aromatic compounds (single and mixed waste streams). In most cases higher temperatures were more effective. The flow rates tested did not appear to have a significant impact on treatment. When compared to conventional radiant heating, microwave treatment is at least as effective, and in some cases (styrene) perhaps more effective. Microwave energy may also offer potential reduced energy costs when compared to some other conventional off-gas treatments.
Future Work
The following are some suggestions for future work:
References