WSRC-MS-2000-00194
How to Recycle Asbestos Containing Materials
C. M. Jantzen and J. B. Pickett
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Abstract
The current disposal of asbestos containing materials (ACM) in the private sector consists of sealing asbestos wetted with water in plastic for safe transportation and burial in regulated land fills. This disposal methodology requires large disposal volumes especially for asbestos covered pipe and asbestos/fiberglass adhering to metal framework, e.g. filters. This "wrap and bury" technology precludes recycle of the asbestos, the pipe and/or the metal frameworks. Safe disposal of ACM at U.S. Department of Energy (DOE) sites, likewise, requires large disposal volumes in landfills for non-radioactive ACM and large disposal volumes in radioactive burial grounds for radioactive and suspect contaminated ACM. The availability of regulated disposal sites is rapidly diminishing causing recycle to be a more attractive option. Asbestos adhering to metal (e.g., pipes) can be recycled by safely removing the asbestos from the metal in a patented hot caustic bath which prevents airborne contamination/inhalation of asbestos fibers. The dissolution residue (caustic and asbestos) can be wet slurry fed to a melter and vitrified into a glass or glass-ceramic. Palex glasses, which are commercially manufactured, are shown to be preferred over conventional borosilicate glasses. The Palex glasses are alkali magnesium silicate glasses derived by substituting MgO for B2O3 in borosilicate type glasses. Palex glasses are very tolerant of the high MgO and high CaO content of the fillers used in forming asbestos coverings for pipes and found in boiler lashing, e.g. hydromagnesite (3MgCO3•Mg(OH)2•3H2O) and plaster of paris, gypsum (CaSO4). The high temperate of the vitrification process destroys the asbestos fibers and renders the asbestos non-hazardous, e.g. a glass or glass-ceramic. In this manner the glass or glass-ceramic produced can be recycled, e.g., glassphalt or glasscrete, as can the clean metal pipe or metal framework.
Introduction
The safe disposal of asbestos containing materials (ACM) in the private sector and at U.S. Department of Energy (DOE) nuclear sites has become problematic. The ACM includes asbestos and fiberglass insulation, boiler lashing, transite, floor tiles, and asbestos covered pipe. The current disposal technique is to seal the ACM and adhering metal (pipe, framework, duct work) in plastic for safe transportation to a burial site. Burial of wrapped asbestos covered pipe and/or duct work necessitates large disposal volumes in regulated disposal sites, e.g. landfills and burial grounds, and expensive removal operations. The availability of regulated disposal sites for ACM has become problematic and expensive.
Technologies have been developed by the private sector and the U.S. Department of Energy’s (DOE) Savannah River Site (SRS) to convert hazardous ACM to a non-hazardous amorphous non-crystalline solid (NCS), glass. Conversion to an NCS eliminates the ACM and fiberglass inhalation hazards which can lead to silicosis and/or asbestosis of the human lung and ultimate death. [1-4].
Asbestos covered pipe comprises the largest volume ACM at SRS. The radioactive and/or suspect radioactive ACM is disposed of in the SRS on-site burial ground. The non-radioactive ACM is disposed of in off-site landfills. In order to be cost effective on a life-cycle basis, the non-hazardous NCS product should provide for a large volume reduction in order to minimize disposal costs for both radioactive (Any radioactive NCS product must also meet the SRS burial ground Performance Assessment (PA) criteria for radionuclides of concern.)and non-radioactive ACM and/or have potential to be recycled (e.g. non-radioactive ACM). The process should also decontaminate the adhering metal, e.g. pipe or duct work, sufficiently that the metal is no longer considered ACM or suspect ACM. In this manner the metal may also be recycled.
It is well known that treatment is more costly (~$330/ton for a 100 ton per day Joule heated melter system) (Per APEX Report on WSRC Subcontract AA07218N (October 13, 1995).) than the current "wrap in plastic and bury" disposal methodologies (~$110/ton) for non-radioactive asbestos. However, treatment eliminates the continuing liability associated with disposal of non-radioactive asbestos-containing wastes in landfills and offers the opportunity for some profit of the recycle material as aggregate in roadways (glassphalt or glasphalt) or in construction (glasscrete) and recycle of the adhering metal. Therefore, an in depth cost evaluation must consider a value added factor for the additional benefits of recycling.
For disposal of radioactive or suspect radioactive asbestos, including asbestos covered pipe in radioactive burial grounds, the disposal costs of the current "wrap in plastic and bury" are $42/ft3 for a low level radioactive burial ground and $760/ft3 for a radioactive (transuranic) burial ground (Westinghouse Savannah River Company 1997 Radiological Worker Training Manual, Rev. 11 (Sept, 1999).). Volume reduction technologies for ACM are, therefore, cost effective, e.g., 99% volume reduction reduces the cost of an equivalent amount of asbestos covered pipe to $0.42/ft3 and $7.60/ft3, respectively.
Asbestos Mineralogy and Health Risks
Asbestos (A commercial term for a group of silicate minerals that readily separate into thin, strong fibers that are flexible, heat resistant, and chemically inert, and are used in a wide variety of industrial products. A mineral of the asbestos group, especially chrysotile (by far the most important), amosite, and crocidolite [R.L. Bates and J.A. Jackson, "Dictionary of Geological Terms", American Geological Institute, Doubleday Publishers, New York, New York (1984).) is a generic designation referring usually to any one of a variety of six different types of naturally occurring mineral fibers [5]. These fibers are extracted during commercial processing from certain varieties of hydrated silicate minerals comprising two mineral families: the serpentines and the amphiboles. The serpentine group contains only one fibrous variety of asbestos called chrysotile (also known as chrysotile asbestos, Mg3[Si2O5](OH)4). The amphibole group contains five fibrous varieties of asbestos known as anthophyllite ((Mg,Fe2+)7[Si8O22](OH)2), amosite ((Fe2+,Mg)7[Si8O22](OH)2), crocidolite (Na2Fe3+2(Fe2+,Mg)3[Si8O22](OH)2), tremolite (Ca2Mg5[Si8O22](OH)2), and actinolite (Ca2(Mg,Fe2+)5[Si8O22](OH)2). Chrysotile asbestos comprised >98% of the world production of asbestos in 1988 while amosite and crocidolite each comprised an additional 1% [5]. The quantities of anthophyllite, actinolite, and tremolite asbestos is insignificant compared to the other three. On a world wide basis the relative incidence of a mesothelioma (a tumor of the layer of squamous cells of the epithelium lining of the pleura, peritoneum, or pericardium (The American Heritage Dictionary, Second College Edition, Houghton Mifflin, Boston, MA (1982).) occuring at an asbestos implant site in rats is 60-75% for crocidolite, ~60% for amosite, and ~58% for chrysotile asbestos [1].
Mineralogic and Chemical Makeup of SRS Asbestos
Samples of SRS transite board, asbestos removed from pipe, and boiler lashing were analyzed by x-ray diffraction (XRD). Table I lists the crystalline species contained in these types of SRS asbestos. The transite board is primarily chrysotile asbestos. The asbestos covered pipe and the boiler lashing are primarily amosite asbestos admixed with large concentrations of magnesium hydroxide carbonate (hydromagnesite).
Table II lists the chemical composition of the SRS asbestos covered pipe analyzed in this study. Since asbestos covered pipe comprises the largest volume ACM at SRS, this material was completely dissolved and analyzed by wet chemical technicques, e.g. Inductively Coupled Plasma Spectroscopy, Atomic Absorption, Ion-Chromotography, and Ion-Selective Electrode (Table II). Since the SiO2 content of this material is relatively low, one can assume that almost all of the silica participates in the formation of the asbestos mineral amosite, (Fe2+,Mg)7[Si8O22](OH)2. Converting all of the SiO2 to moles of amosite, and assuming that the amosite is all Fe2+ allows one to calculate the amount of amosite in the asbestos pipe covering: 25% of the pipe covering is amosite asbestos. Mass balance calculations demonstrate that the remaining 75% of the pipe covering is hydromagnesite a hydroxide carbonate species and not an asbestos mineral. Therefore, 75% of the asbestos covered pipe is a non-asbestos mineral filler. Note that the boiler lashing also contains hydromagnesite filler. During high temperature conversion of the ACM material and filler, the CO3-, OH- and H2O contributions (~47%) of the converted materials will be vaporized as steam and CO2 gas.
Table I. Mineralogic Content of SRS Asbestos
|
SRS Asbestos Type |
Mineralogic Content |
Chemical Makeup |
|
Transite |
Clinochrysotile Asbestos |
Mg3 [Si2O5](OH)4 |
|
Pipe Covering |
Amosite Asbestos |
(Fe2+,Mg)7 [Si8O22](OH)2 |
|
Boiler Lashing |
Amosite Asbestos |
(Fe2+,Mg)7 [Si8O22](OH)2 |
Table II. Chemical Composition of Asbestos Covering SRS Pipe
(~25 wt% Amosite Asbestos and ~75 wt% Hydromagnesite)
|
Oxide |
Wt% |
Oxide |
Wt% |
|
Al2O3 |
0.35 |
P2O5 |
0.08 |
|
CaO |
1.29 |
SiO2 |
8.07 |
|
Cr2O3 |
0.03 |
TiO2 |
0.02 |
|
Fe0 |
10.10 |
ZnO |
0.01 |
|
K2O |
0.06 |
Cl |
0.156 |
|
MgO |
33.74 |
SO4 |
0.185 |
|
MnO |
0.13 |
CO2 |
28.55* |
|
Na2O |
0.01 |
H2O + OH- |
18.42* |
|
NiO |
0.01 |
SUM |
101.21 |
* the amount of CO2, H2O, and OH- present was calculated by a mass
balance from the primary mineralogic species identified by XRD in Table I.
A similar wet chemical and x-ray diffraction analysis of the black tar paper covering the pipe indicates that it is SiO2 rich (~25wt%) material with ~40wt% organics. The remaining chemistry is dominated by ~10wt% H2O and OH-, ~10 %MgO, and 10% Na2O, and 5% Fe2O3. The inorganic fraction is composed of chrysotile (3MgO,2SiO2,2H2O), Talc (H2O, 3MgO, 4 SiO2), a clinochlore (4H2O, 5MgO, Al2O3, 3SiO2), and a trace of muscovite mica (2H2O, K2O, 3Al2O3,SiO2). Only one of these minerals, chrysotile, is an asbestos mineral which can only be present at ~1.5wt% based on the amount of MgO available (There are 7 moles of MgO in chrysotile so 10wt%/7 = 1.42 wt% chrysotile).
Hot Caustic Dissolution Of Asbestos
A caustic-acid-caustic dissolution methodology was developed for dissolution of contaminated fiberglass HEME/HEPA filters from the SRS High Level Waste glass melter off-gas system [6-7]. These filters are housed in metal filter frames. The fiberglass was found to convert to silica gel after the first dissolution step in 5 wt% NaOH at 90°C for 48 hours [7]. After the NaOH dissolution, the HEME/HEPA filter frames were found to be clean. Air sparging [8] of the tank accelerated the reaction and was necessary to ensure the total conversion. The dissolution solutions (NaOH) and the dissolution residues (silica gel) are glass forming elements that are sent back to the DWPF melter and vitrified. A patent was applied for and granted [7] that umbrellas the use of the DWPF dissolution methodology on all fibrous high silica containing materials, including asbestos.
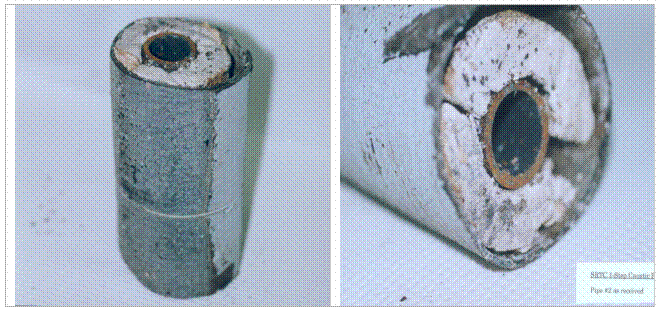
Since asbestos covered pipe is the largest volume of ACM and the most difficult to deal with at the SRS, the one step NaOH dissolution methodology was tested on a 7" section of an asbestos covered pipe without separating the asphalt cover or the metal wires that tightly hold the asphalt around the ACM and the pipe (Figure 1). The hot 5 wt% NaOH solution was not sparged, not bubbled, not stirred, and 2000mL of solution was used on a 7" long segment of pipe.
The 48 hour dissolution in hot 5%NaOH did nothing to the asphaltic material covering the pipe. This was confirmed by a second x-ray diffraction analysis, e.g. the same phases were present in the asphaltic material before and after the 48 hour treatment. Although the tar paper contains ~40% organics it can be vitrified along with the ACM since the tar paper comprises only a small amount (by weight or volume) of the overall material being processed.
The white ACM material was partially converted after the 48 hour dissolution. All of the hydromagnesite had been converted and the amosite asbestos had been partially converted. X-ray diffraction of the converted ACM indicated that magnesium hydroxide (Mg(OH)2) sodium carbonate (Na2CO3) were present as decomposition products of the non-asbestos phase, hydromagnesite. X-ray diffraction of the amosite asbestos were broad instead of sharp indicating that the crystalline structure of this phase had been partially destroyed, e.g. became amorphous. The NaOH solution was analyzed and found to contain high concentrations of Al and Si indicating that the silicate based asbestos minerals were indeed dissolving. The NaOH plus dissolved ACM was the consistency of

Figure 1. The 7" long as-received sections of pipe showing how the tar paper is loosely wired to the ACM covered pipe. Note how the ACM is in 2 clam-shell or c-shaped sections with a 1/4" to 1/2" gap between the sections of ACM and between the ACM and the pipe. This allows the hot NaOH solution to interact with the ACM down the entire length of pipe without removing the tar paper.
"lumpy oatmeal" and could easily be fed to a melter for vitrification. The final vitrification completes the conversion of the ACM to an NCS.
If the asphaltic material covering the pipe is loosened, pierced, or removed to allow better reaction between the ACM and the solution, and/or if the tank is agitated slightly the ACM will convert more completely in the dissolution tank before being pumped to a melter. If the pipe is not sufficiently clean, a rinse in HNO3 can sufficiently decontaminate the iron pipe as the surface of the pipe reacts with the acid to form various iron oxides and hydroxides. The iron oxides and hydroxides spall off releasing the adhering ACM so that the pipe can be recycled. These smaller amounts of ACM and iron oxides/hydroxides can be admixed with the converted ACM from the caustic only process during the vitrification process.
Vitrification of Dissolution Solution and Residues
The ACM composition before NaOH dissolution is given in Table II. Since ~47 wt% of the ACM volatilizes during vitrification, the composition of the ACM contains ~60 wt% MgO on an oxide (glass forming) basis. The composition of the ACM after dissolution in 5wt% NaOH for 48 hours contains between 16-27 wt% MgO on an oxide (glass forming) basis. The high MgO content makes dissolution of this material into borosilicate glass difficult, e.g. there is a high potential for the ACM to not completely dissolve in borosilicate glass.
In 1939, Riedel in Czechoslovakia invented a series of low thermal expansion Palex type glasses by replacing the B2O3 in borosilicate type glasses with MgO. These glasses (including Palex 5/13 in Table III below) were produced commercially [9]. The Palex glasses are known to form at a maximum of 8 wt% alkali oxides (Na2O + K2O), 5 wt% Al2O3 or ZnO, and a maximum of 15 wt% MgO. This glass was used as fire-proof and heat resistant laboratory glassware. It was the forerunner of our current day Pyrex laboratory glassware. The Palex glasses are also very tolerant of BaO and CaO and can acccomodate the gypsum (CaSO4) plaster often found associated with asbestos covered pipe. Palex glasses with BaO substituted for MgO/CaO were made by Schott Glass and were used for the manufacture of baby milk bottles [10].
The Palex glasses are more tolerant of large concentrations of MgO and CaO than the borosilicate glasses as they are essentially alkali magnesium silicate glasses. Up to 60 wt% ACM was accommodated into the Palex glasses in this study. Since the commerical Palex type glass melts in excess of 1450°C (composition 5/13 in Table III), 4-7 wt% Li2O was substituted in some glass formulations per the SRTC Lithia Additive Melting Process (LAMP™) (patent pending) to lower the melt temperature to ~1150°C (see Table III).
The Palex type glasses have the flexibility to be melted at high temperatures (³ 1250°C) using cheaper K2O or Na2O glass forming additives if volatile hazardous or radioactive species are not of concern. Alternatively, the Palex glasses can be melted at lower temperatures (~1150°C) using more expensive Li2O glass forming additives if volatile radionuclides or hazardous constituents are of concern or if the melter materials of construction necessitate lower temperatures. In all cases totally amorphous glass and/or a mixture of amorphous glass and a non-asbestos mineral spinel were formed at either 1150°C or 1250°C indicating that all of the ACM was converted to an amorphous form and all of the hazards from inhalation of asbestos fibers removed.
The volume reduction achieved by vitrification (Figure 2) can be expressed as:
[1-(Volglass/VolACM+pipe)]*100 = [1-(14.13cc/4703cc)]*100= 99.7%
Alternatively, one could calculate the volume reduction without including the contribution from the pipe, e.g. 400 grams of ACM made 31.3 grams of glass product. Since amosite asbestos has a density of 2.85 g/cc and the measured waste glass has a density of 2.37g/cc, the relative volumes of waste and product can be calculated as 140 cc of ACM and 17.7 cc of glass. The volume reduction is then expressed as: [1-(Volglass/VolACM)]*100 = 90.6%
Table III. Asbestos Containing Palex Glasses Developed by SRTC
|
GLASS OXIDE |
PALEX 5/13 |
ACM PALEX |
ACM PALEX |
ACM PALEX |
ACM PALEX |
ACM PALEX |
|
Al2O3 |
0.00 |
0.43 |
0.43 |
0.43 |
0.22 |
0.22 |
|
CaO |
0.00 |
0.74 |
0.74 |
0.74 |
0.37 |
0.37 |
|
Cr2O3 |
0.00 |
0.30 |
0.30 |
0.30 |
0.15 |
0.15 |
|
Fe203 |
0.00 |
19.72 |
19.72 |
19.72 |
9.86 |
9.86 |
|
K2O |
3.50 |
0.16 |
0.16 |
4.16 |
0.08 |
7.08 |
|
Li2O |
0.00 |
--- |
4.00 |
--- |
7.00 |
--- |
|
MgO |
15.00 |
13.72 |
13.72 |
13.72 |
6.86 |
6.86 |
|
MnO |
0.00 |
0.17 |
0.17 |
0.17 |
0.09 |
0.09 |
|
Na2O |
3.50 |
17.48 |
17.48 |
17.48 |
8.74 |
8.74 |
|
NiO |
0.00 |
0.13 |
0.13 |
0.13 |
0.07 |
0.07 |
|
P2O5 |
0.00 |
0.06 |
0.06 |
0.06 |
0.03 |
0.03 |
|
SiO2 |
79.00 |
47.01 |
43.01 |
43.01 |
66.51 |
66.51 |
|
TiO2 |
0.00 |
0.06 |
0.06 |
0.06 |
0.03 |
0.03 |
|
ZnO |
0.00 |
0.02 |
0.02 |
0.02 |
0.01 |
0.01 |
|
OXIDE SUM |
101.00 |
100.00 |
100.00 |
100.00 |
100.00 |
100.00 |
|
WASTE LOADING (Wt%) |
N/A |
60 |
60 |
60 |
30 |
30 |
|
Additives |
40 SiO2 |
36 SiO2 |
36 SiO2 |
63 SiO2 |
63 SiO2 |
|
|
Amorphous |
Yes |
No |
Yes |
Yes |
Yes |
|
|
MELT TEMP (°C) |
³ 1450 |
1250 |
>1250 |
1250 |
1150 |
1250 |
Conclusions
Vitrification of ACM (asbestos covered pipe) after dissolution in NaOH at 90°C was demonstrated in SRTC using the patented reference process [7] for dissolution/vitrification of radioactively contaminated fiberglass filters for the Defense Waste Processing Facility (DWPF). In the DWPF the fiberglass is dissolved in the hot NaOH in a large metal basket and the tank is fitted with sparging rings to accelerate the dissolution. Once clean, the adhering filter framework which is metal is lifted out of the tank in the metal basket. The dissolved fiberglass filter gelatinous residue and the NaOH dissolution solution are fed directly as a slurry to the DWPF melter for vitrification. The totally wet processing of ACM in hot NaOH solution minimizes inhalation hazards of ACM during treatment.

Figure 2. Volume reductions achievable with the caustic-only or caustic-acid-caustic dissolution methodologies coupled with Joule Heated Ceramic Melting (JHCM).
The melter is operated at an atmosphere slightly less than atmospheric to contain any particulate asbestos fibers and any radioactive or hazardous constituents. The NaOH dissolution solution is one of the glass forming chemical fluxes needed to vitrify the ACM. If the ACM is non-radioactive, the treated ACM material can be poured out of the melter into a disposal can and allowed to cool or water quenched into large vats for recycle. The process is semi-continuous. The resulting ACM product is a totally amorphous non-hazardous glass that is free of undissolved ACM. If the ACM is radioactive or suspect radioactive, the glass atomistically bonds any radioactive or hazardous constituents that were either in or on the original ACM material including Pb paint.
For non-radioactive non-hazardous ACM, the resulting glass can enter the recycle market along with the decontaminated metal pipe, duct work or filter frames. For radioactive or suspect radioactive ACM a volume reduction of 99.7% is achievable which minimizes disposal/burial costs considerably. The pipe is rendered non-radioactive and can be recycled.
Acknowledgement
This paper was prepared in connection with work done under Contract No. DE-AC09-96SR18500 with the U.S. Department of Energy.
References