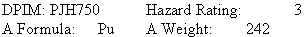
WSMS-SAE-01-0204
Toxicological versus Radiological Hazards of 239Pu
D. K. Craig
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Introduction
Plutonium is frequently referred to as "the most toxic substance known to man". While there are other chemicals that cause more immediately serious health consequences, plutonium does have the lowest permissible levels for any of the radioactive elements. Concentration limits for plutonium and its compounds are based on its radiotoxicity, specifically carcinogenicity, not on its chemical toxicity. The specific activity of 239Pu is 61.3 m Ci/mg, the inverse of which is 16.3 m g/m Ci1, its half-life being 24,400 years. The derived air concentration (DAC) for 239Pu is 2 x 10-12 m Ci/cc = 2 x 10-6 m Ci/m3, which is based on a committed effective dose equivalent of 50 mSv (5 rem)2.
Chemical Toxicity of Plutonium
In terms of mass per unit volume, the DAC for 239Pu of 2 x 10-6 m Ci/m3 is equal to 3.26 x 10-5 m g/m3. Shorter-lived plutonium isotopes have even smaller mass concentration limits. The most restrictive time-weighted average (TWA) threshold limit values (TLVs) listed3 are 0.001 mg/m3 for calcium chromate, and 0.0005 mg/m3 (i.e., 5 x 10-1 m g/m3) for strontium chromate. Both of these limits are based on the potent carcinogenic potential of these chemicals, so they form a good basis for comparison with the alpha-emitting plutonium isotopes. The TLV-TWA is 0.002 mg/m3 for both the soluble salts of platinum, and hexachlorobenzene, and 0.001 mg/m3 for calcium chromate (as Cr). The TLV booklet gives "Notice of intended changes (for 2001)" for "Beryllium [7440-41-7] and compounds, as Be" from 0.002 to 0.0002 mg/m3 (i.e., 2 x 10-1 m g/m3) for the inhalable fraction, also based on its carcinogenic potential. No other chemical substances have lower workplace limits than this new value for Be.
The two relevant plutonium entries in SAX5 are duplicated in the appendix.
Discussion and Conclusions
Clearly, the radiation-based limit for 239Pu is several (four) orders of magnitude less than that of the chemical with the most restrictive workplace concentration limit. A four order-of-magnitude increase in the DAC for 239Pu would result in a committed effective dose equivalent of 50,000 rem (i.e., an average dose of 1000 rem per annum), enough to cause acute radiation death in at least some people. Radiation dose rates of this magnitude make it impossible to test the non-radiation biological effects: animals die from the radiation effects long before toxic effects can manifest themselves.
Any overt toxicity of plutonium and its compounds would be overwhelmed by their radiological effects.
References
Appendix
Plutonium
Properties:
A silvery, radioactive metal; chemically reactive. Melting point: 641º, boiling point: 3232º, density: 19.816 @ 20º/4º.
Safety Profile
An extremely poisonous radioactive material. The permissible levels for plutonium are the lowest for any of the radioactive elements. This is occasioned by the concentration of plutonium directly on bone surfaces, rather than the more uniform bone distribution shown by other heavy elements. This increases the possibility of damage from equivalent activities of plutonium and has led to the adoption of the extremely low permissible levels given. Radiation Hazard: Artificial isotope 238Pu, T0.5 = 86 Y, decays to radioactive 234U by alphas of 5.5 MeV. Artificial isotope 239Pu, T0.5 = 24,000 Y decays to radioactive 235U by alphas of 5.1 MeV. Artificial isotope 240Up, T0.5 = 6600 Y decays to radioactive 236Pu (Neptunium Series), T0.5 = 13 Y decays to radioactive 241Am by betas of 0.02 MeV. Artificial isotope 242Pu, T0.5 = 3.8 × 105 Y decays to radioactive 238U by alphas of 4.9 MeV. Ignites in air as low as 135ºC. Explosive reaction with carbon tetrachloride. Particles exposed to air and moisture may ignite spontaneously. Corrosion products are usually pyrophoric. When heated to decomposition it emits toxic and radioactive fumes of Pu. See also Plutonium Compounds.
Plutonium Compounds
Safety Profile
The toxicity of plutonium compounds is based first upon the very high radiotoxicity
of the plutonium atom and secondly upon whatever atoms or combinations of atoms
they might contain. Very dangerous! Any disaster which causes quantities of
plutonium or plutonium compounds to be scattered about the environment will
cause great ecological stress and render areas of the land unfit for public
occupancy. Long-term storage in plastic containers is not recommended, as the
alpha particles can cause stress cracks and there is a potential for leakage.
See also Plutonium.