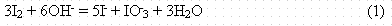
WSRC-TR-99-00270
I-129 Desorption from SRS Water Treatment Media
from the Effluent Treatment Facility and the
F-Area Groundwater Treatment Facility
D. I. Kaplan, S. M. Serkiz, and N. C. Bell
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department
of Energy and its contractors, in paper, from: U.S. Department of Energy, Office
of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062,
phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Keywords: Radionuclide Speciation and Transport, Waste Form Leaching, Anion Exchange Resin, Mercury Exchange Resin, Activated Carbon, Iron Hydroxide Sludge, I-129, Performance Assessment
Executive Summary
Recent performance assessment (PA) modeling of the Intermediate Level Vault in E-Area located on the Savannah River Site (SRS) was used as the basis to set waste acceptance criteria (WAC) for radionuclides at this facility. This PA modeling effort used waste leaching and transport factors derived largely from literature data for I-129, a primary risk driver in this study area. A distribution coefficient (Kd value), defined as the activity in the solid phase divided by the activity in the aqueous phase, of only 2.0 mL/g was used to calculate I-129 leaching from the waste and to establish the WAC in the revised draft PA. Because some of the sorbents were designed to remove contaminants from the aqueous phase, the actual I-129 Kd of these materials is expected to be greater than the general literature values.
The objective of this study was to provide waste-specific I-129 Kd values that would permit the PA to "take credit" for the sorptive behavior of four waste materials from the Effluent Treatment Facility and the F-Area Groundwater Treatment Facility. Because the PA considers the desorption of radionuclides from waste materials over thousands of years, additional attention was directed at quantifying the change in I-129 Kd values as a function of time, or more specifically leachate volume. I-129 Kd values were measured by column and batch methods, each method providing unique information about I-129 desorption behavior from these materials. The column experiments provided information about I-129 desorption as a function of time/leachate volume; however, this technique does not provide conservative (lowest possible) Kd values. The batch experiments provided conservative estimates of Kd values, but only limited information about temporal changes in Kd values. Liquid in the form of cement simulant (high pH and ionic strength), to represent the vault environment, and in the form of simulated acid rain water (pH 3, low ionic strength, and poorly buffered), to represent a trench environment, was put in contact with the waste samples. 20L of these liquids were passed through the columns, representing >5000 pore volumes, the amount of liquid that is expected (is being modeled) to pass through the waste in about 1100 years.
Initial I-129 activity (in units of pCi/g dry weight) in the ETF Carbon was 495, F-WTU Dowex 21K was 296, in ETF GT-73 was 48 and F-WTU Sludge was <6.7. Due to the lack of I-129 activity in the F-WTU Sludge, Kd values could not be measured on this waste. Column studies revealed that I-129 Kd values from the waste generally increased sharply as the amount of leachate passed through the column increased. For example, increases in Kd values by greater than an order of magnitude were measured simply between the first and second 1-L sampling of leachate from the column studies. This increase was attributed to the more readily extractable species of I-129 being leached from waste materials first, leaving the more strongly sorbed I-129 species on the waste. Batch Kd values were generally consistent with column studies, tending to be greater than the 1-L leachate column Kd values, but less than the 8-L leachate column Kd values. Reasonably conservative I-129 Kd values in a cement system, based primarily on the batch Kd values, were:
The acid-rain simulant desorbed appreciably less I-129 (i.e., had greater Kd values) than the cement simulant. Reasonably conservative I-129 Kd values in a simulated acid rain environment were:
It is important to note that these latter estimates are based on few laboratory data.
These studies provide technically defensible evidence for employing I-129 Kd values appreciably greater than the default values of 2 mL/g that were used in the revised draft PA. A number of issues were identified during these studies. This work indicates that the chemical environment in trenches may be more stable for iodine sequestration than in concrete vaults. Additionally, it is likely that the present default I-129 Kd value of 0.6 mL/g used in the geological subsurface may be overly conservative, especially in the naturally acid environment of the SRS. The inclusion of "getters," geological materials that can sequester I-129, at the disposal site may provide extra protection against I-129 migration. Finally, additional work is needed to establish the long-term stability of the various waste forms, especially the organic resin materials. Research directed at these issues will provide needed technical defensibility and will improve modeling accuracy that may increase WAC limits.
Introduction
The introduction section describes the problem, an overview of iodine geochemistry, objectives, scope, and the general approach used in the study.
Problem Definition
Recent performance assessment (PA) modeling of the E-Area Waste Disposal Vaults (DOE 1994, 1998) was used as the basis to set waste acceptance criteria (WAC) for radionuclides at this facility. This PA modeling effort used the distribution coefficient (Kd value) construct to quantify aqueous I-129 chemical interactions with the waste and the geological materials. The Kd is defined as the concentration in the solid phase divided by the concentration in the aqueous phase. The I-129 Kd values used in the most recent PA calculations were 0.6 mL/g for soil and 2 mL/g for the Intermediate Level Vault (ILV) waste (DOE 1998). Failure of the cement vault was described as occurring in three steps. During the first 575 years, the physical integrity of the cement vault remained intact. During the following 475 years, the cement formed contiguous cracks through out the vault. After this period, after 1050 years, the vault was assumed to have completely crumbled. The aqueous chemistry never changes however; it remains strongly influenced by the presence of the cement, i.e., it has a high pH and high ionic strength. Because of the low-Kd values and conceptual model used, I-129 was predicted to be released into the subsurface as a large pulse over a relatively short time period. From these calculations, Waste Acceptance Criteria (WAC) were established to meet the 0.5 pCi/L Maximum Contaminant Level for I-129. Based on these WAC limits, several SRS waste streams, including activated carbon and GT-73 resin from the Effluent Treatment Facility (ETF) and Dowex-21K resin from the F-Area Water Treatment Unit (WTU) at the Groundwater Remediation Facility are not permitted to be disposed at this facility. These wastes are presently in temporary storage until WAC limits are modified or an alternative disposal location can be identified.
Geochemical Behavior of Iodine
There are 24 known isotopes of I with 18 of these isotopes having half-lives of less than 1 day. The only stable isotope is I-127. Its average natural abundance in geologic materials is 5 mg/kg (Gu and Schulz 1991) and its concentration in uncontaminated surface waters is typically <1 mg/L (Stumm and Morgan 1981). The isotope of concern for long-term disposal at the Savannah River Site is I-129, which has a half-life of 1.7 x l07 years.
Aqueous Speciation
Iodine usually exists in fresh water in the minus one oxidation state as iodide (I-) (Whitehead 1984). In alkaline and marine environments, iodine usually exists in the VII oxidation state as iodate (IO3-) (Whitehead l984). The other oxidation states of iodine, III and V, are much less frequently found in nature. Iodide is likely to be the dominant iodine species in the Savannah River Site subsurface because its domain of predominance extends throughout the pH scale, completely covering a large part of the stability domain of water (Figure 1; Ticknor and Cho 1990). Oxidation of I2 to produce IO-3, the second most abundant form of iodine in aqueous systems, is easily accomplished in basic solution by the reaction:
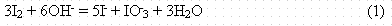
(Cotton and Wilkinson l972). Thus, the IO-3 form of iodine is likely dominant in well-oxidized, high-pH systems as may exist in the cement vaults of the ILV. Iodide and IO-3 were the most commonly detected species in rainwater collected after the Chernobyl accident (Muramatsu et al. 1990). Iodide and IO-3 tend to exist as free ions, but the complexes they do form are generally the most soluble of all halide complexes.
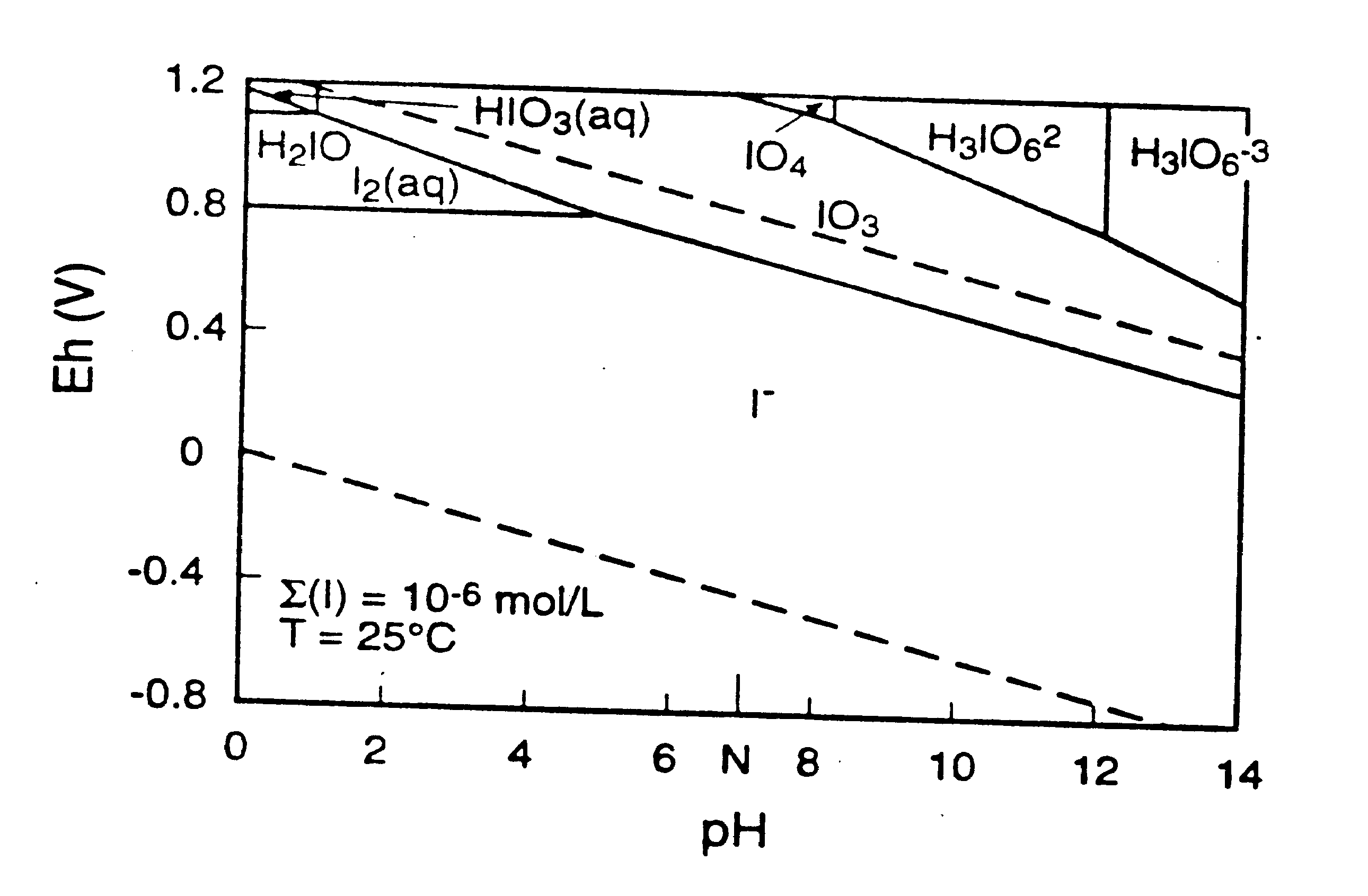
Figure 1. Eh and pH Diagram for Iodine/Water System (Ticknor and Cho l990)
Precipitation and Coprecipitation
Precipitation and coprecipitation are not likely to be a dominant reaction path for iodine in the cement vault environment. Iodide, in comparison with the other halides, forms especially weak complexes with metal ions as a result of its large size (0.22-nm ionic radius, Langmuir l979). The metals with which I- and IO-3 form sparingly soluble compounds, Ag, Ba, Hg, Pb, and Pd (Pourbaix l966) exist in nature at very low concentrations. The low concentrations of I in the contaminant plume will likely exist as either free species or as highly soluble complex species. One important possible exception, is the ETF Gt-73 resin, which contains Hg in its structure. Should this Hg be released in the presence of the I-129, these elements will likely form extremely insoluble compounds. The concentration of Hg leached from the ETF Gt-73 resin by the TCLP standard EPA test is less than regulatory limits. However, only trace amounts, less than the TCLP limit, may be sufficient to immobilize significant amounts of I-129.
Sorption
Two types of reactions between anions and inorganic solids are recognized: specific adsorption and nonspecific adsorption. Specific adsorption refers to incorporation of anions as a ligand in the coordination shell of an adsorbent, while nonspecific adsorption refers to adsorption of anions by simple coloumbic (electrostatic) interactions with positive charges. Iodine anions are believed to sorb primarily through nonspecific, anion-exchange reactions on mineral surfaces (Gu and Schulz 1991) and through specific adsorption on organic substances (Walters and Winchester 1971). Nonspecific sorption may occur at the localized positive charges that occur on (1) Fe- and Al- oxide surfaces, (2) edges of aluminosilicate clay surfaces where the oxygen atoms are not fully coordinated by Al or Si atoms, and 3) on amine and amino groups of organic substances. These positive charges, which increase with decreasing pH, attract anions electrostatically. Whitehead (1973), for example, reported that sorption of I- by soils was associated with both soil organic matter and Fe and Al oxides, with the oxides increasingly important under more acidic conditions. The maximum amounts sorbed by two surfaces were at pH 6.6. At this pH, the amounts of I- sorbed were found to be closely related to the contents of organic matter but not to Fe- or Al- oxides or clay. At pH<5, the removal of Fe- and Al-oxides resulted in a marked reduction in I- sorption. Whitehead (1974) further observed that freshly precipitated ferric- and Al-oxides sorbed substantial amount of I- from solutions of pH <5.5 but the amount decreased to zero as the pH approached 7. Presumably, this trend reflects the presence of an increasing amount of positive charge (anion-exchange capacity) on the amphoteric oxide surfaces at lower pH levels.
Ticknor and Cho (1990) studied the interaction of I- and IO-3 over a pH range of 7.5 to 8.0 with a number of minerals including calcite, chlorite, epidote, goethite, gypsum, hematite, kaolinite, bentonite, muscovite, and quartz. No I- sorption was detected from any of the solutions on any of the minerals. Iodate was removed from solution to a somewhat greater extent then I-. Bentonite, calcite, gypsum, and muscovite absorbed no IO3-. Muramatsu et al. (1990) reported that neither I- nor IO3- sorbed to quartz sand. The authors concluded that the low I- and IO-3 sorption was the result of the low anion-exchange capacities of the minerals at the high pH of the systems in the investigation. Recently, Kaplan et al. (1999) showed that some naturally occurring 2:1 phyllosilicate minerals have the ability to sorb large amounts of iodide. Kd values >60 mL/g were measured for illites. Even at pH >9, iodide Kd values of these illites were >20 mL/g. Subsurface Hanford sediment with a pH of 8 had Kd values that average 3 mL/g and ranged from 0.1 to 10 mL/g.
Objectives and Scope
The objective of this study was to quantify I-129 desorption of four waste materials and to provide reasonably conservative Kd values for future modeling efforts. Since the ILV PA considers dose thousands of years in the future, additional attention was directed at evaluating how I-129 desorption changed as a function of time. The scope of this work involved evaluating four waste materials (F-WTU Dowex 21K, F-WTU Sludge, ETF Carbon, and ETF GT-73) under two aqueous conditions (acid rain and cement simulants) by two different experimental protocols (static batch and dynamic flow column experiments).
General Approach
A series of laboratory studies were initiated to provide waste-specific desorption parameters for activated carbon and GT-73 resin from the ETF and Dowex-21K resin and iron hydroxide sludge from the F-Area WTU clarification process. These desorption parameters can then be used to update PA modeling calculations with waste-stream specific data.
Column and batch leaching studies with both acid and cement leaching solutions were employed in this work. A standard acid leaching solution (ASTM D 4874-95) was used: 1) as a means of comparing data from this study to other results generated using this standard extraction technique, and 2) to provide an indication of the influence of acid rain on I-129 leaching from these wastes. It was also intended to use these acid-leaching results as a preliminary evaluation of the iodine-leaching behavior of these wastes in a shallow land disposal setting. The cement leaching simulant is designed to mimic the chemistry of the leachate in water infiltrating through the concrete vaults.
In the column studies, samples of each of the waste streams were leached in duplicate with both the acid and cement leaching solution and the effluent was collected in one-liter aliquots (representing approximately 200 pore volumes). Tests were run until a total of 20 liters of effluent were collected and selected aliquots were analyzed for I-129 activity. Solid phase I-129 activities were also measured before and after the test.
Additionally, solids prior to and after column leaching were subjected to a single batch extraction. In contrast to the column leaching experiments, where the leaching solution was in contact with the waste for ~0.8 min, samples were allowed to equilibrate for a week. These batch studies were designed to provide a maximum leach rate, or conservative Kd value, for the wastes and to evaluate if the rate of desorption changed as a function of leachate volume passed through the waste materials. During the leaching tests, it was anticipated that a finite fraction of the I-129 would quickly and readily desorb (i.e., weakly sorbed fraction) from the waste materials with the first volume of leaching solution, leaving behind more strongly sorbed species that desorbed at a slower rate. Stated differently, it was expected that the I-129 Kd values would increase as more I-129 was leached.
Materials and Methods
This section describes the materials and methods used to evaluate the leaching behavior of iodine from ion exchange/sorbent materials used in the F-Area Groundwater Treatment Facility and the Effluent Treatment Facility. Included in this section are descriptions of collection and characterization of process sorbent materials, the general experimental approach, column experiment description, batch experiment description, and sample analyses/quality assurance.
Sorbent Material Collection and Characterization
Environmental Restoration personnel from WSRC collected and transported F-Area WTU spent Dowex 21K ion exchange resin (ADTECHS’s Anion Resin 4% crosslinked) and iron hydroxide sludge (iron-aluminum hydroxide precipitate formed during the F-WTU neutralization process) to SRTC on 2/2/99. The samples were not preserved and were stored at room temperature prior to analysis. ETF activated carbon and GT-73 resin samples were remnants of material from previous analyses by the Analytical Development Section (ADS) of WSRC.
General Experimental Approach
Sorbent materials for these tests were taken from the ion exchange columns at the F-Area WTU and the ETF. Leaching of iodine was evaluated with both column and batch experiments and with both a simulated acid rain leaching solution and a simulated cement pore water leaching solution. All samples were analyzed for I-129 by the Analytical Development Section (ADS), Measurement Technology Department, of the Savannah River Technology Center.
All bench-scale testing was conducted using established test methods and the general approach contained in the following procedures.
Schematics of batch and column experiments are presented in Figures 2 and 3, respectively. In order to screen rapidly all sorbent materials, and to reduce cost and secondary waste production, modifications to the ASTM procedures (column dimensions, mass of sorbent, and flow rate) were necessary. These modifications are presented below in the detailed description of the laboratory protocol used in this study.
Column Experiment
Column studies were conducted to provide a measure of the change in I-129 desorption as a function of volume of leachate. The merits of column and batch methods of measuring Kd values are discussed in Section 3.2. The procedure adapted from the ASTM methods listed above follows.
The I-129 concentration data were then used to calculate Kd values using Equations 1 or 2,
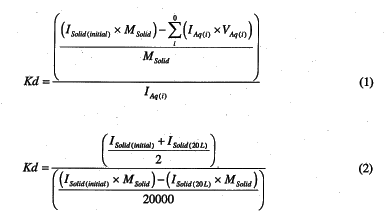
where ISolid(initial) and ISolid(20L) are the I-129 activity in the solid material at the start and after 20-L of leachate had been passed through the material (pCi/g), respectively, IAq(i) is the I-129 activity in the ith 1-L leachate aliquot (pCi/mL), MSolid is the solid mass, and VAq(i) is the aqueous volume of the ith 1-L leachate aliquot (mL).
It is important to note that Kd values calculated from Equation 1 require I-129 concentration data of the solid and aqueous phases, whereas Kd values calculated from Equation 2 require only I-129 concentration data of the solid phase. It is not clear which of these two equations provide more accurate estimates of Kd values. The most common equation used for calculating Kd values is Equation 1 (ASTM 1995) since most researchers do not take the time or resources to measure contaminant concentration in the solid phase. In Equation 1, the I-129 concentration of the solid phase (the numerator) is not measured directly. Instead, it is estimated by subtracting the total activity leached into the aqueous phase from the initial activity in the solid phase. In Equation 2, the I-129 concentration of the aqueous phase (the denominator) is not measured directly. Instead, it is estimated by subtracting the final concentration in the solid (in our case, the solids concentration after 20 L of leachate had been passed through the solids) from the initial solids concentration. The Kd calculated by Equation 2 is actually an averaged value that reflects the Kd that existed at the mid-point of the leachate experiment, namely the Kd that existed at the 10-L leachate volume. The 20000 divisor is the volume in mL of the leachate and 2 in the numerator is to calculate the average of the initial and final activity in the solids. All Kd values were reported on a dry weight basis.
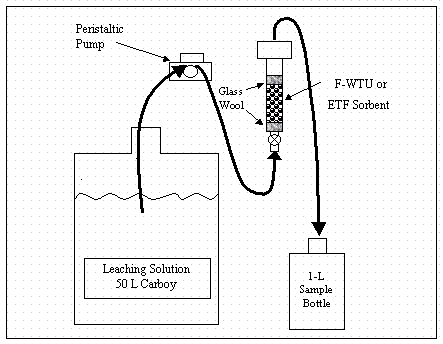
Figure 2. Column experiment schematic
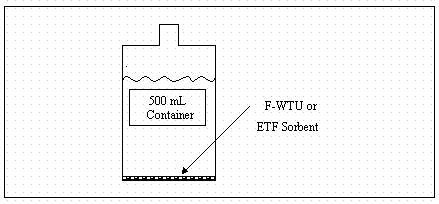
Figure 3. Batch experimental schematic
Batch Experiment
I-129 Kd values for pre- and post-leach (after 20-L leachate) waste materials in acid rain or cement simulant solutions were measured by the following procedure that is a modification of standard methods (ASTM 1991, 1993).
Five grams of sorbent material and 475-mL leaching solution were placed into 500-mL plastic bottles and gently mixed for 30 seconds.
Batch leaching experiments were allowed to equilibrate for 7 days, during which time the sample bottles were gently mixed once per day for 30 seconds.
Following the 7-day equilibration period, leaching solutions were filtered (0.45 m m) and submitted to ADS for analyses of I-129.
Kd values were calculated using Equation 3,

where ISolid(final) is the I-129 activity in the solid at the end of the equilibration period (pCi/g), MSolid is the mass of the solid (g), VAq is the volume of the aqueous phase (mL), and IAq(final) is the aqueous I-129 activity at the end of the equilibration period (pCi/mL). All Kd values were reported on a dry weight basis.
Sample Analysis and Quality Assurance
Column effluent samples and sorbent materials (pre- and post-leach) from leaching tests were analyzed by the Analytical Development Section (ADS), Measurement Technology Department, Savannah River Technology Center. Details of analytical procedures and QA requirements for these analyses can be obtained from their offices. Briefly, between 450 and 750-mL aliquots of aqueous sample were subjected to a silver iodide precipitation method to separate any iodide in the matrix from other radionuclides. The precipitate was then analyzed for as long as 2 days using a LOAX HPGe gamma spectroscopy detector. A blank deionized water sample was analyzed along with the experimental samples as a negative control. This control provided information about background I-129 contamination resulting from laboratory activities. After the gamma analysis the precipitate was analyzed by neutron activation analysis (NAA) to determine the levels of stable iodide carrier in the precipitate. The recovery of the iodide carrier was used to correct the gamma spectroscopy results for the I-129 recovery. The standard QA practices described in the WSRD QA Manual 1Q were followed throughout this study.
Results and Discussion
Column Experiment
To reduce cost and to adhere to schedule, only a small subset of the 1-L aliquots collected from the column leachates were analyzed for I-129 activity. The first (0- to 1-L effluent), fourth (3- to 4-L effluent), and eighth (7- to 8-L effluent) 1-L aliquots were analyzed for I-129 activity. (The twentieth aliquot, a potential sample, was not analyzed due to conflicts with schedule and cost.) Additionally, the I-129 activity was measured in the solids at the start of the experiment and after 20-L of leachate had been passed through them.
The initial I-129 activity in the waste materials evaluated in this study varied several orders of magnitude (Table 1). The ETF Carbon contained the most I-129 activity. The F-WTU Sludge contained no detectable I-129 activity, consequently no Kd measurements could be made using this material.
Table 1. Initial I-129 activity and moisture content in samples used in study.
|
Material |
This Study |
Previous Studies(a) |
|||
|
Moisture Content |
I-129 Activity |
I-129 Activity (pCi/g |
Average
|
||
|
(pCi/g |
(% Uncertainty at 1 s )(b) |
||||
|
ETF Carbon |
37.12 |
311 |
4.95 |
495 |
87/201(c) |
|
F-WTU Dowex 21K |
47.37 |
156 |
5.17 |
296 |
119 |
|
ETF GT-73 |
65.24 |
16.7 |
11.8 |
48 |
51 |
|
F-WTU Sludge |
84.35 |
<1.04 |
-- |
<6.7 |
0.89 |
During this study, 20-L of simulated waste was passed through the columns. This volume represents about 5000 pore volumes (~10 g waste material, ~1.3 g/mL bulk density, and ~0.5 mL/mL porosity). This volume also represents about 1100 years in the repository (assuming the PA Darcy flux of 4 cm/yr for the first 1050 years and then a 40 cm/yr flux for subsequent years (DOE 1994, 1998); the cross-sectional area of the column was 3.14 cm2).
The leach rate of I-129 varied with the type of waste material (Figure 4). The activity decreased most rapidly for the ETF Carbon sample: from 494.6 pCi/kg to 18.6 pCi/g, a decrease of 96%, after 20 liters of leachate had passed through the material. The I-129 activity in the F-WTU Dowex 21K resin decreased from 296.4 to 72.6 pCi/g, a decrease of 75%, after 20 liters of leachate had passed through the material. The I-129 activity in the ETF GT-73 resin decreased from 48.0 to 40.4 pCi/g, a decrease of 16%, after eight liters of effluent had passed through the material (the activity in the 20-L solid sample was not accurately measured). Additional details are presented in Table A2 in the Appendix.
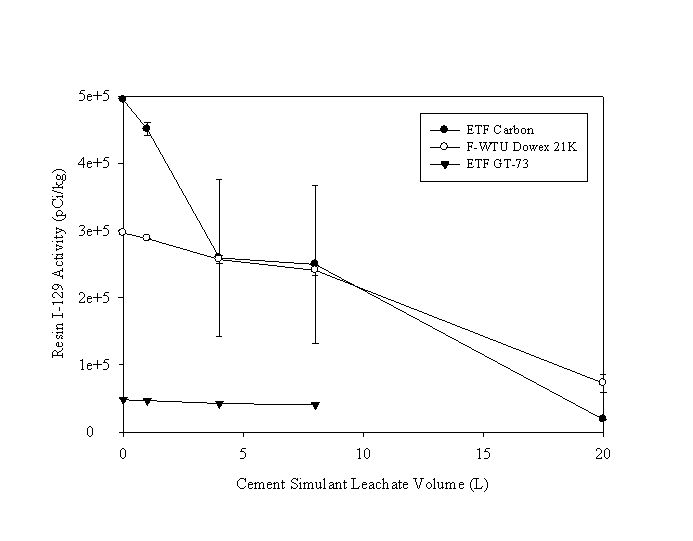
Figure 4. Change in I-129 activity in waste materials
as a function of cement simulant leachate volume.
(The mean and standard deviation of 2 replicates; error bars are smaller than
symbols for many means.)
The data for two replicates in Figure 4 were used with Equation 1 to calculate Kd values (Table 2). The Kd values tended to increase as the leach volume increased. This may be attributed to the more readily leachable I-129 being removed during the initial pore volumes. The higher I-129 activity leached into the aqueous phase (IAq(i) in Equation 1) would cause Kd value to decrease. Over time, only the more strongly held I-129 remained on the solids, resulting in less I-129 being leached into the aqueous phase. For the most part, the standard deviations between the two replicates were small, suggesting that the experimental and analytical error were small and perhaps more importantly, that the leaching behavior of different sample aliquots were similar.
Table 2. Column study Kd values with cement
leachate simulant based
on aqueous and solid I-129 concentration data.(a)
|
Leachate |
ETF Carbon |
F-WTU Dowex 21K |
ETF GT-73 |
|||||||||
|
Volume |
Average |
Std. Dev. |
Average |
Std. Dev. |
Average |
Std. Dev. |
||||||
|
(L) |
(mL/g) |
(mL/g) |
(mL/g) |
|||||||||
|
1 |
745 |
168 |
3127 |
433 |
3541 |
573 |
||||||
|
4 |
10061 |
5982 |
11978 |
4764 |
17032 |
8784 |
||||||
|
8 |
35730 |
1047 |
10982 |
118 |
15970 |
6627 |
||||||
Kd values were also calculated using the I-129 solid concentration data (Equation 2) for the samples that had 20-L of leachate passed through them (Table 3). These Kd values, calculated from different data than was used to calculate the Kd values in Table 2, were similar in magnitude to the 1-L leachate Kd values in Table 2. Again, the standard deviations of the two replicates were quite small, suggesting good experimental technique and uniformity in I-129 leaching behavior between two subsamples.
Table 3. Kd values (mL/g) based on solid I-129 activities
after 20-L of cement leachate
or acid rain simulant were passed through waste material(a).
|
Material |
Cement Leachate Kd (mL/g) |
Acid Rain Kd (mL/g) |
||
|
ETF Carbon |
1524 ± 27 |
6851 ± 143 |
||
|
F-WTU Dowex 21K |
2962 ± 165 |
7448 ± 304 |
||
|
ETF GT-73 |
NA(b) |
21757 ± 7442 |
||
Some standard procedures for leaching contaminants from materials (e.g., ASTM 1993) require that an acid extract be used. Since the environment in the cement waste form is not acidic, it was elected to substitute a more representative aqueous phase. The aqueous phase used in a Kd measurement can have a significant impact on sorption and desorption processes (Sposito 1984). For purposes of comparison to the standard method, we elected to measure the I-129 activity in a limited number of waste materials before and after passing 20-L of acid-rain simulant through them. As can be seen in Table 3, the acid rain Kd values were appreciably greater than the cement leachate Kd values. This result underscores the detrimental effect that the cementitious environment has on the leaching chemistry of iodine. Additional data from the column experiments are presented in the Appendix (Tables A3 and A4).
Batch Experiment
I-129 Kd values were measured from batch experiments to provide a comparison with the Kd values measured from the column experiments. These two methods measure two different processes, yet each has advantages and disadvantages for estimating Kd values for the ILV PA. The column experiment permits measurement of the Kd value under the proper solid to liquid ratio. The solid to liquid mass ratio in the column experiments was generally about 1:0.4, whereas in the batch experiments this ratio was 1:95.
The problem with conducting a column experiment at very slow flow rates, such as 4 mL/yr, is that it is difficult, perhaps impossible, to obtain such low flow rates and it is difficult to generate a sufficient volume of liquid for analytical purposes. Column experiments conducted at flow rates faster than is expected in the field do not provide the proper solid-liquid contact time. In the column experiments conducted for this study, the contact time of the water as it passed through the column was ~0.8 min (0.01 kg solid/column, flow rate of 5e-3 L/min, bulk density of 1.3 kg/L, porosity of 0.5 L/L). This contact time is many orders of magnitude less than that expected under field conditions. The problem with a shorter contact time is that it would tend to overestimate actual Kd values. This would occur because the concentration of the I-129 in the leachate, the denominator of the measured Kd value (Equation1), would be reduced due to mass-transfer kinetic limitations of I-129 desorption from the solid to the liquid phase. To provide a contact time that is more similar to expected field conditions, batch experiments were conducted with contact times (equilibration times) of 7 days. It was anticipated that the greater contact time would more closely simulate actual field conditions and would permit a greater amount of I-129 to be released from the solids, thereby resulting in a lower Kd value.
Batch Kd values were conducted with "as received" samples and samples that had been "aged" during the column study by passing 20-L of cement leachate through them. It was expected that the batch Kd measurements of the "as received" samples would provide the most conservative Kd values due to the general tendency for solids to release contaminants more readily in early leaching volumes. Thus, it was expected that the 20-L leached samples would have greater Kd values than the "as received" samples.
The batch Kd values (Table 4) tended to be within the range of values measured by the column studies (Tables 2 and 3). As discussed above, it was expected that the "aged" samples would have greater Kd values than the "as received" samples (0-L leachate volume passed through column prior to batch test). This was not observed consistently in this data set (Table 4). This can in part be attributed to radiological detection limitations of the "aged" aqueous samples. Batch Kd values measured in the acid-rain simulant were greater than the Kd values measured in the cement-leachate simulant. This finding is consistent with the column Kd values which showed that the acid-rain Kd values were greater than the cement-leachate Kd values (Table 3). The trends of the ETF Carbon Kd values are difficult to explain. First, the extremely high Kd value for the acid solution, > 181,836 mL/g, is inconsistent with all the other Kd values collected with this material. Secondly, it is difficult to explain why the 20-L "aged" samples had: 1) a lower Kd value than the "as received" samples and 2) a lower Kd value by a factor of 2.62 than any other Kd value measured (Tables 2 and 3).
Table 4. Batch I-129 Kd values
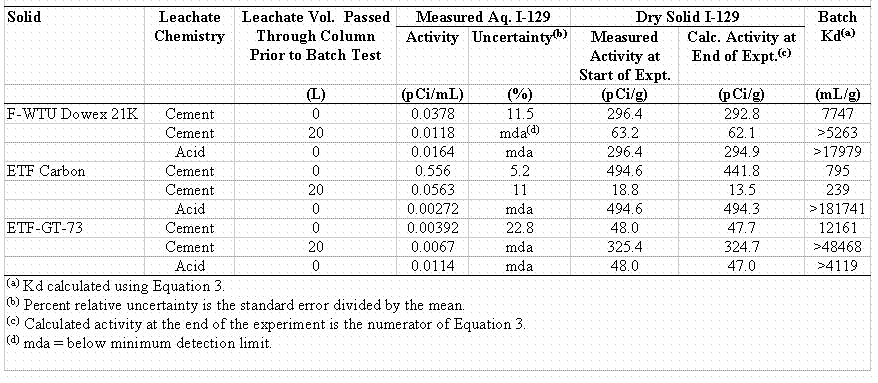
Reasonably Conservative Kd Estimate
The lowest measured Kd value was used as the basis for the reasonably conservative Kd estimate for the F-WTU Dowex 21K and the ETF GT-73 resins (Table 5 and 6). We elected not to follow this easily defensible strategy for the selection of the reasonably conservative Kd estimate for the ETF Carbon because the lowest Kd value is questionable insofar that it does not follow well-established trends based on fundamental surface chemistry. This Kd value is less than the Kd value of the "as received" sample. It is also 2.62 times smaller than the second smallest value measured by the column experiments, an experimental method, as discussed earlier, that is expected to generated larger Kd values. Given the uncertainty of this value, our estimation of a reasonably conservative Kd value for ETF Carbon was based on the second lowest measured Kd value, 627 mL/g. The reasonably conservative Kd estimates were rounded to the nearest 100 mL/g to reflect the accuracy we feel these values represent.
Table 5. All Kd values measured in cement leachate simulant
for the F-WTU
Dowex 21K resin, ETF Carbon, and ETF GT-73 resin.
|
Expt. |
Vol. Leachate Passed Through Solid (L) |
Equation Used in Kd Calc. |
Rep |
Table in This Document Where Data is Presented |
F-WTU Dowex 21K Kd (mL/g) |
ETF Carbon Kd (mL/g) |
ETF GT-73 |
|
Column |
1 |
1 |
1 |
2 |
3433 |
864 |
3136 |
|
Column |
1 |
1 |
2 |
2 |
2821 |
627 |
3946 |
|
Column |
4 |
1 |
1 |
2 |
15347 |
14292 |
23243 |
|
Column |
4 |
1 |
2 |
2 |
8609 |
5831 |
10821 |
|
Column |
8 |
1 |
1 |
2 |
11066 |
36470 |
11284 |
|
Column |
8 |
1 |
2 |
2 |
10899 |
34990 |
20656 |
|
Column |
20 |
2 |
1 |
3 |
2845 |
1505 |
na |
|
Column |
20 |
2 |
2 |
3 |
3078 |
1543 |
na |
|
Batch |
0 |
3 |
1 |
4 |
7747 |
795 |
12161 |
|
Batch |
20 |
3 |
1 |
4 |
>5263 |
239 |
>4868 |
Table 6. Reasonably conservative I-129 Kd estimates for three waste materials in a cementitious environment
|
Waste Material |
Kd (mL/g) |
|
F-WTU Dowex 21K |
2800 |
|
ETF Carbon |
600 |
|
ETF GT-73 |
3100 |
All the acid-rain simulant Kd values measured in this study are presented in Table 7. Based on these limited number of measurements, reasonably conservative I-129 Kd estimates were selected based on the lowest values (Table 8). It is important to note that these reasonably conservative estimates are based on few measurements and therefore are less defensible than the cementitious estimates.
Table 7. All Kd values measured in acid-rain simulant for
the F-WTU Dowex 21K resin, ETF Car
on, and ETF GT-73 resin.
|
Expt. |
Vol. Leachate Passed Through Solid (L) |
Equation Used in Kd Calc. |
Rep |
Table in This Document Where Data is Presented |
F-WTU Dowex 21K Kd (mL/g) |
ETF Carbon Kd (mL/g) |
ETF GT-73 Kd (mL/g) |
|
Column |
20 |
2 |
1 |
3 |
7233 |
6750 |
27,019 |
|
Column |
20 |
2 |
2 |
3 |
7663 |
6952 |
16,494 |
|
Batch |
0 |
3 |
1 |
4 |
>17979 |
>181741 |
>4119 |
Table 8. Reasonably conservative I-129 Kd estimates for three
waste
materials in an acid rain (pH 3) simulant environment.
|
Waste Material |
Kd (mL/g) |
|
F-WTU Dowex 21K |
6800 |
|
ETF Carbon |
7400 |
|
ETF GT-73 |
10,000 |
Conclusions
Batch and column experiments were conducted to measure I-129 Kd values for four waste materials: F-WTU Dowex 21K resin, F-WTU Sludge, ETF Carbon, and ETF GT73 resin. No detectable I-129 was measured in the F-WTU Sludge and therefore no Kd values could be measured for this material. For the remaining waste materials, a wide range of I-129 Kd values was generated for each waste material. For the F-WTU Dowex 21K under cementitious conditions, the Kd values ranged from 2821 to 11,066 mL/g. For the ETF Carbon, the Kd values ranged from 239 to 36,470 mL/g. For the ETF GT-73, the Kd values ranged from 3136 to >48,468 mL/g. The wide range can be attributed to the wide range of experimental conditions, and the fact that three techniques were used to measure the Kd values. The column experiments are expected to yield non-conservative Kd values, where the batch experiments, especially those of the "as received" samples, would provide a closer measure of the most conservative value. Based on the data and justification presented in this report, the reasonably conservative Kd estimates are:
The acid-rain simulant desorbed appreciably less I-129 (i.e., had greater Kd values) than the cement simulant. Reasonably conservative I-129 Kd values in a simulated acid rain environment are 6800 mL/g for F-WTU Dowex 21K, 7400 mL/g for ETF Carbon, and 10,000 mL/g for ETF GT-73. It is important to note that these latter estimates are based on few laboratory data.
These studies provide technically defensible evidence for employing I-129 Kd values appreciably greater than the default values of 2 mL/g used in the latest draft of the PA (DOE 1998). A number of issues were identified during these studies. This work indicates that the chemical environment in trenches may be more stable for iodine than in cement vaults. Additionally, it is likely that the present default I-129 Kd value of 0.6 mL/g used in the geological subsurface may be overly conservative, especially in the naturally acid environment of the SRS. The inclusion of "getters," geological materials that can sequester I-129, at the disposal site may provide extra protection against I-129 migration. Finally, additional work in needed to establish the long term stability of the various waste forms, especially the organic resin materials. Research directed at these issues will provide needed technical defensibility and will improve modeling accuracy that may increase WAC limits.
References
Appendix: Supplemental Data
Table A1. Previously reported I-129 activities and moisture content in samples used in study(a)
| Solid |
I-129 Activity
(pCi/g moist wt ± 1 s ) |
Detection Limit
(pCi/g moist wt) |
Moisture
|
|
|
F-WTU filtercake/sludge |
0.25 ± 0.49 |
|||
|
F-WTU filtercake/sludge |
2.42 ± 1.01 |
0.80 |
47.5 |
|
|
F-WTU filtercake/sludge |
0.46 ± 0.86 |
1.05 |
43.8 |
|
|
F-WTU filtercake/sludge |
0.29 ± 0.60 |
0.71 |
||
|
F-WTU filtercake/sludge |
0.99 ± 0.74 |
0.81 |
||
|
F-WTU filtercake/sludge |
0.94 ± 0.87 |
1.05 |
||
|
Average F-WTU filtercake/sludge |
0.89 |
|||
|
F-WTU Dowex 21K |
119 ± 14.6 |
1.37 |
||
|
ETF GT-73 Resin 12/98 |
51.1 |
NA |
||
|
ETF Carbon A, 0-12" (Organic Removal Vessel #5) |
75.7 |
3.5 |
||
|
ETF Carbon A, 13-24" (Organic Removal Vessel #5) |
78.6 |
4.2 |
||
|
ETF Carbon B, 0-12" (Organic Removal Vessel #5) |
96.8 |
5.1 |
||
|
ETF Carbon B, 13-24" (Organic Removal Vessel #5) |
97.6 |
5.1 |
||
|
Average Carbon Vessel #5 |
87.2 |
|||
|
ETF Carbon 0-12" (Organic Removal Vessel #9) |
177 |
12.0 |
||
|
ETF Carbon 12-24" (Organic Removal Vessel #9) |
318 |
19.2 |
||
|
ETF Carbon 24-36" (Organic Removal Vessel #9) |
139 |
9.1 |
||
|
ETF Carbon 36-48" (Organic Removal Vessel #9) |
170 |
11.0 |
||
|
Average Carbon Vessel #9 |
201 |
|||
|
Overall Avg for Carbon Vessels #5 & #9 |
144 |
|||
(a) Data taken from: Lucha 1997 (Lucha, C. 1997. Low Level Waste Stream Characterization. SWE-WSCF-1997-0030. Westinghouse Savannah River Company, Aiken, SC.)
Table A2. I-129 activity in the solids as a function of the
volume of cement
leachate simulant volume passed through column(a).
|
Leachate |
ETF Carbon |
F-WTU Dowex 21K |
ETF GT-73 |
|||||||||
|
Volume |
Average |
Std. Dev. |
Average |
Std. Dev. |
Average |
Std. Dev. |
||||||
|
(L) |
(pCi/kg) |
(pCi/kg) |
(pCi/kg) |
(pCi/kg) |
(pCi/kg) |
(pCi/kg) |
||||||
|
0 |
494593 |
0 |
296409 |
0 |
48044 |
0 |
||||||
|
1 |
450749 |
9758 |
288089 |
798 |
46845 |
164 |
||||||
|
4 |
259331 |
116646 |
256934 |
5305 |
42468 |
235 |
||||||
|
8 |
249694 |
117239 |
240804 |
7644 |
40441 |
63 |
||||||
|
20 |
18579 |
320 |
72651 |
13336 |
na |
na |
||||||
Table A3. I-129 concentrations in solutions generated from the column experiments.
|
Waste Material |
Leaching |
Leaching |
Rep |
Lab ID |
ADS LIMS # |
I-129 Activity |
Average |
Uncertainty |
|
Solution |
Vol. |
# |
I-129 Activity |
at 1 s |
||||
|
(L) |
(pCi/L) |
(pCi/L) |
(%) |
|||||
|
F-WTU Dowex 21K |
Cement |
1 |
1 |
C-1-1 |
126871 |
8.41E+01 |
9.30E+01 |
8.4% |
|
F-WTU Dowex 21K |
Cement |
1 |
2 |
C-1B-1 |
126872 |
1.02E+02 |
6.7% |
|
|
F-WTU Dowex 21K |
Cement |
4 |
1 |
C-1-4 |
126873 |
1.70E+01 |
2.32E+01 |
18.5% |
|
F-WTU Dowex 21K |
Cement |
4 |
2 |
C-1B-4 |
126874 |
2.94E+01 |
8.4% |
|
|
F-WTU Dowex 21K |
Cement |
8 |
1 |
C-1-8 |
126875 |
2.22E+01 |
2.19E+01 |
16.1% |
|
F-WTU Dowex 21K |
Cement |
8 |
2 |
C-1B-8 |
126876 |
2.16E+01 |
13.6% |
|
|
F-WTU Filter Cake |
Cement |
1 |
1 |
C-2-1 |
126877 |
<3.51E+00 |
<6.30E+00 |
mda |
|
F-WTU Filter Cake |
Cement |
1 |
2 |
C-2B-1 |
126878 |
<9.09E+00 |
mda |
|
|
F-WTU Filter Cake |
Cement |
4 |
1 |
C-2-4 |
126879 |
<6.95E+00 |
<6.18E+00 |
mda |
|
F-WTU Filter Cake |
Cement |
4 |
2 |
C-2B-4 |
126880 |
<5.40E+00 |
mda |
|
|
F-WTU Filter Cake |
Cement |
8 |
1 |
C-2-8 |
127150 |
<5.99E+00 |
<6.06E+00 |
mda |
|
F-WTU Filter Cake |
Cement |
8 |
2 |
C-2B-8 |
127151 |
<6.14E+00 |
mda |
|
|
ETF Activated Carbon |
Cement |
1 |
1 |
C-3-1 |
127152 |
5.30E+02 |
6.19E+02 |
6.7% |
|
ETF Activated Carbon |
Cement |
1 |
2 |
C-3B-1 |
127153 |
7.08E+02 |
6.1% |
|
|
ETF Activated Carbon |
Cement |
4 |
1 |
C-3-4 |
127154 |
2.39E+01 |
3.61E+01 |
8.2% |
|
ETF Activated Carbon |
Cement |
4 |
2 |
C-3B-4 |
127155 |
4.82E+01 |
9.3% |
|
|
ETF Activated Carbon |
Cement |
8 |
1 |
C-3-8 |
127156 |
<9.12E+00 |
not available |
-- |
|
ETF Activated Carbon |
Cement |
8 |
2 |
C-3B-8 |
127157 |
7.58E+00 |
14.9% |
|
|
ETF GT-73 |
Cement |
1 |
1 |
C-4-1 |
127615 |
1.49E+01 |
1.34E+01 |
8.30% |
|
ETF GT-73 |
Cement |
1 |
2 |
C-4B-1 |
127616 |
1.19E+01 |
13.20% |
|
|
ETF GT-73 |
Cement |
4 |
1 |
C-4-4 |
127617 |
1.82E+00 |
2.88E+00 |
17.90% |
|
ETF GT-73 |
Cement |
4 |
2 |
C-4B-4 |
127618 |
3.94E+00 |
mda |
|
|
ETF GT-73 |
Cement |
8 |
1 |
C-4-8 |
127619 |
3.58E+00 |
2.77E+00 |
mda |
|
ETF GT-73 |
Cement |
8 |
2 |
C-4B-8 |
127620 |
1.96E+00 |
mda |
Table A4. Solid I-129 concentrations in materials from the column experiments.
|
Waste |
Pre or Post |
Leaching |
Wet Wt. Solid in Column |
rep # |
Lab ID |
ADS LIMS # |
I-129 Conc. |
Uncertainty |
|
F-WTU Dowex 21K |
Pre |
-- |
-- |
1 |
1-Pure |
3-125954 |
1.56E+02 |
5.17 |
|
F-WTU Dowex 21K |
Post |
Cement |
10.3 |
1 |
C-1-solid |
3-126885 |
3.33E+01 |
5.9 |
|
F-WTU Dowex 21K |
Post |
Cement |
10.9 |
2 |
C-1B-solid |
3-126886 |
4.32E+01 |
5.0 |
|
F-WTU Dowex 21K |
Post |
Acid |
10.2 |
1 |
1-solid |
3-125951 |
9.21E+01 |
5.37 |
|
F-WTU Dowex 21K |
Post |
Acid |
10.7 |
2 |
1B-solid |
3-125953 |
9.73E+01 |
5.15 |
|
F-WTU Sludge |
Pre |
-- |
-- |
1 |
2-Pure |
3-125946 |
1.04E+00 |
MDA |
|
F-WTU Sludge |
Post |
Cement |
25.7 |
1 |
C-2-solid |
3-126888 |
1.64E+00 |
MDA |
|
F-WTU Sludge |
Post |
Cement |
24.5 |
2 |
C-2B-solid |
3-126889 |
1.08E+00 |
MDA |
|
ETF Carbon |
Pre |
-- |
-- |
1 |
3-Pure |
3-125950 |
3.11E+02 |
4.95 |
|
ETF Carbon |
Post |
Cement |
11.4 |
1 |
C-3-solid |
3-126892 |
1.18E+01 |
8.9 |
|
ETF Carbon |
Post |
Cement |
11.1 |
2 |
C-3B-solid |
3-126893 |
1.15E+01 |
6.4 |
|
ETF Carbon |
Post |
Acid |
11.6 |
1 |
3-solid |
3-125948 |
2.06E+02 |
5.27 |
|
ETF Carbon |
Post |
Acid |
11.8 |
2 |
3B-solid |
3-125949 |
2.10E+02 |
5.38 |
|
ETF GT-73 |
Pre |
-- |
-- |
1 |
4-Pure |
3-125943 |
1.67E+01 |
11.8 |
|
ETF GT-73 |
Post |
Cement |
16.3 |
1 |
C-4-solid |
3-126895 |
1.13E+02(a) |
5.5 |
|
ETF GT-73 |
Post |
Cement |
15.8 |
2 |
C-4B-solid |
3-126896 |
9.74E+01(a) |
5.6 |
|
ETF GT-73 |
Post |
Acid |
15.1 |
1 |
4-solid |
3-125936 |
1.45E+01 |
8 |
|
ETF GT-73 |
Post |
Acid |
14.9 |
2 |
4B-solid |
3-125942 |
1.32E+01 |
8.1 |
(a) Unreasonable value because the concentration of I-129 in the solids after leaching was greater than the I-129 concentration prior to leaching (for comparison, see the ETF GT-73/Pre I-129 concentration value immediately above these two values).