
WSRC-TR-2001-00331
Chemical and Radiological Toxicity of Uranium and its Compounds
D. K. Craig
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Introduction
The following information about the toxicity of uranium and its more common compounds is extracted and slightly edited, from SAX1.
Safety Profile
(Uranium is) a highly toxic element on an acute basis. The permissible levels for soluble compounds are based on chemical toxicity, whereas the permissible body level for insoluble compounds is based on radiotoxicity. The high chemical toxicity of uranium and its salts is largely shown in kidney damage, which may not be reversible. Acute arterial lesions may occur after acute exposures.The most soluble uranium compounds are UF6, UO2(NO3)2, UO2Cl2, UO2F2, and uranyl acetates, sulfates, and carbonates.
Some moderately soluble compounds are UF4, UO2, UO4, (NH4)2U2O7, UO3, and uranyl nitrates. The rapid passage of soluble uranium compounds through the body tends to allow relatively large amounts to be absorbed. Soluble uranium compounds may (also) be absorbed through the skin.
The least soluble compounds are high-fired UO2, U3O8, and uranium hydrides and carbides. The high toxicity effect of insoluble compounds is largely due to lung irradiation by inhaled particles. This material is transferred from the lungs of animals quite slowly.
(Uranium is) a very dangerous fire hazard in the form of a solid or dust when exposed to heat or flame.
Standards and Recommendations
Occupational Exposure Guides

Emergency Exposure Guides
Temporary Emergency Exposure Limits (TEELs)5 are used by DOE as concentration limits for emergency planning and management for chemicals lacking Emergency Response Planning Guidelines (ERPGs)6. For most uranium compounds, the TEEL values, as uranium, are:
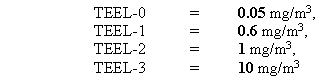
Exceptions are uranyl nitrate (TEEL-2 = 0.6 mg/m3), and uranium hexafluoride, for which there are ERPGs6. Like uranium dioxide (UO2), the solubility of uranyl nitrate (UO2(NO3)2 can vary, depending upon its formation.
Occupational Radiation Exposure Limits
Isotopic data are extracted from the Handbook of Chemistry and Physics7. These data for naturally occurring uranium isotopes are summarized in Table 1. The isotopic composition of any uranium compound is a function of its history. "Natural" uranium, for which the composition is shown in the second column, has been shown to be a toxicity rather than a radiation hazard, although this is somewhat dependent upon the solubility in body fluids of the particular chemical compound involved.
Table 1. Properties of and Limits for the Isotopes of Natural Uranium
|
Uranium Isotope |
% Natural Abundance |
Atomic Weight |
Half-life |
SA |
DAC8 |
|
234 |
0.0055 |
234.040945 |
2.45x105 y |
6.24 |
8.01E-5 |
|
235 |
0.720 |
235.043922 |
7.04x108 y |
2.16x10-3 |
0.278 |
|
238 |
99.2745 |
238.050784 |
4.46x109 y |
3.36x10-4 |
1.786 |
|
Natural |
1.775 |

Health Hazards
Background
In order for substances like uranium to pose a hazard, they must enter the body in the first place. To do so, they must be inhaled, ingested, or absorbed through the skin. To be an inhalation hazard, they must at least comprise "inhalable" particles or, in the case of insoluble compounds, "respirable" particles. For insoluble compounds of uranium, only the respirable fraction of the inhaled material is of importance, whereas for soluble compounds, it is the inhalable fraction that will contribute the bulk of the dose. It is important to evaluate the source term, not only to ascertain the compound(s) released, but also to determine the potential particle size distribution (e.g., inhalable and respirable fractions).
There are three separate listings for uranyl nitrate in both SAX1 and RTECS9:
Elemental Uranium, CASRN = 7440-61-1, MW = 238.00,.SG = 19.05.
Uranium(IV) oxide, CASRN = 1344-57-6, MW = 270.03, MF = UO2, SG =
7.3.
Both uranyl nitrate and uranyl nitrate hexahydrate are moderately soluble in water and in the body. This means that if ingested or inhaled, they will readily be transported to the rest of the body. They can also be absorbed through the skin. ICRP10 considers uranyl nitrate to be in Class D (i.e., retention half-time less than 10 days).
Uranium metal is insoluble in water. Uranium(IV) oxide is reported by SAX1 to be moderately soluble in body fluids, unless it is high-fired. ICRP10 considers uranium(IV) oxide to be in Class Y (i.e., half-time retention in the pulmonary region greater than 100 days).
Chemical Toxicity
The TLV documentation11 gives a comprehensive review of the observed toxicity of uranium compounds in both experimental animals and humans. This review cites 75 references. A number of human epidemiological studies are reviewed. Current TLV-TWA and TLV-STEL values are based largely on the findings from these worker-exposure studies. The high toxicity of soluble compounds of uranium is largely manifest in irreversible kidney damage. Blood disorders have also been observed in uranium industry workers. Uranyl nitrate clearly falls into this category. Elemental uranium, on the other hand, is largely insoluble, but since it is soluble in aqueous HCl, some ingested material could be absorbed from the stomach. Its main effect when inhaled is to the lung.
Radiation Hazards
SAX states: "The high toxicity effect of insoluble compounds is largely due to lung irradiation by inhaled particles. This material is transferred from the lung rather slowly." Uranium(IV) oxide qualifies as an insoluble compound. The derived air concentration limits are based on either a committed dose equivalent of 5 rems (or 05 Sv), or an organ dose limit of 50 rems per year, whichever is more limiting. The most restrictive DAC values were used, namely, those for biological retention times of less than 10 days (Class D). This would apply to uranyl nitrate (DAC = 1.775 x 394/238 mg/m3 = 2.94 mg/m3), but not to high-fired uranium oxide (Class Y). The DAC for insoluble uranium is thirty-fold lower than that for soluble uranium. Therefore, uranium(IV) oxide has a workplace concentration limit, in units of mass per unit volume, of
DAC = 1.775/30 x 270/238 = 0.067 mg/m3.
The comparable value for soluble uranyl nitrate is
DAC = 1.775 x 394/238 = 2.94 mg/m3
Discussion
Toxicologically speaking, there is a difference between uranium oxide and uranyl nitrate. Uranyl nitrate, including the hexahydrate form, is soluble in water and moderately soluble in the body. This means that if ingested or inhaled, it will readily be transported to the rest of the body. It can also be absorbed through the skin. Its high toxicity is largely manifest in irreversible kidney damage. Uranium oxide, on the other hand, is largely insoluble. Only respirable particles deposited in the pulmonary region of the lung are retained for long enough for the radiological consequences to manifest themselves. Since UO2 is soluble in aqueous HCl, some ingested material could be absorbed from the stomach.
The above DACs for soluble uranyl nitrate and insoluble uranium(IV) oxide may be compared with the PEL-TWA is 0.05 mg/m3, the TLV-TWA of 0.2 mg/m3, and TLV-STEL of 0.6 mg/m3 for both soluble and insoluble uranium compounds. Only NIOSH distinguishes between soluble and insoluble uranium compounds, i.e., REL-TWA = 0.05 mg/m3 for soluble compounds, and REL-TWA = 0.2 mg/m3 and REL-STEL of 0.6 mg/m3 for insoluble compounds. Both the PEL-TWA and the REL-TWA are based on potential carcinogenesis from lung irradiation. These limits are close to the DAC of 0.067 mg/m3 calculated above.
The IDLH for both soluble and insoluble compounds of uranium is 10 mg/m3, which is the source of the TEEL-3 value for uranium and all its compounds. The PEL-TWA gives the TEEL-0 value of 0.05 mg/m3, and the TLV-STEL gives the TEEL-1 of 0.6 mg/m3. These limits may be multiplied by 1.655 (i.e., 394/238) for uranyl nitrate, and 1.134 (i.e., 270/238) for uranium(IV) oxide. The DAC of about 3 mg/m3 for uranyl nitrate is larger than all the limits except the IDLH (Immediately Dangerous to Life or Health value from NIOSH),
Conclusions
The concentration of uranyl nitrate required to deliver the radiation dose limit for soluble uranium compounds is larger than the toxicity-based concentration limits. Therefore, for soluble uranium compounds, health consequences of exposure are primarily due to their chemical toxicity. For insoluble compounds of uranium, health consequences (e.g., fibrosis and/or carcinogenesis of the lung) are primarily due to irradiation of pulmonary tissues from inhaled respirable particles.
References