WSRC-TR-2001-00063
Mercury Removal, Methylmercury Formation,
and Sulfate-Reducing Bacteria
Profiles in Wetland Mesocosms Containing Gypsum-Amended
Sediments and Scirpus californicus
J. K. King and J. B. Gladden
Westinghouse Savannah River Company
Aiken, SC 29808
S. M. Harmon
University of South Carolina
Columbia SC 29208
T. T. Fu
Skidaway Institute of Oceanography
Savannah, GA 31411
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Abstract
A pilot-scale model was constructed to determine if a wetland treatment system (WTS) could effectively remove low-level mercury from an outfall located at the Department of Energy’s Savannah River Site. Site-specific hydrosoil was planted with giant bulrush, Scirpus californicus, and surface amended with gypsum (CaSO42-) prior to investigating the biogeochemical dynamics of sediment-based sulfur and mercury speciation. On average, the pilot WTS decreased total mercury concentrations in the outfall stream by 50%. Transformation of mercury to a more "bioavailable" species, methylmercury, was also observed in the wetland treatment system.
Methylmercury formation in the wetland was ascertained with respect to sediment biogeochemistry and Scirpus californicus influences. Differences in sulfate-reduction rates (SRR) were observed between mesocosms that received additional decomposing Scirpus matter and mesocosms that were permitted growth of the submerged macrophyte, Potamogeton pusillus. Relative abundance measurements of sulfate-reducing bacteria (SRB) as characterized using oligonucleotide probes were also noticeably different between the two mesocosms.
A positive correlation between increased sulfide, dissolved total mercury, and dissolved methylmercury concentrations was also observed in porewater. The data suggest that soluble mercury-sulfide complexes were formed and contributed, in part, to a slight increase in mercury solubility. Observed increases in methylmercury concentration also suggest that soluble mercury-sulfide complexes represent a significant source of mercury that is "available" for methylation. Finally, a volunteer macrophyte, Potamogeton pusillus, is implicated as having contributed additional suspended particulate matter in surface water that subsequently reduced the pool of dissolved mercury while also providing an environment suitable for demethylation.
Key words: methylmercury, wetlands, sulfide, sulfate-reduction
Introduction
The use of constructed wetlands technology to remove metals from urban runoff, acid-mine drainage, and industrial outfalls has been well documented (Scholes et al., 1998; Huddleston et al., submitted; Gillespie et al., 2000; Hawkins et al., 1997; Mitsch and Wise, 1998; Dombeck et al., 1998; Dunbabin and Bowmer, 1992). However, little information exists regarding the use of wetland-based systems to remove "low-level" inorganic mercury (Hg2+) from industrial discharges.
Reductions of parts per trillion mercury in surface waters has become a major initiative since recently collected human toxicology data suggests that allowable mercury concentrations in fish be lowered to 0.30 mg-kg-1(USEPA, 2001; NRC, 2000). As a result, mercury total maximum daily loads (TMDLs) of 2.83 ng-l-1 to surface waters comprising the Savannah River Basin in the southeastern United States have been proposed by U.S. Environmental Protection Agency -Region IV (USEPA, 2000). These heightened restrictions on mercury release to the watershed have spurred efforts to find means of removing "low-level" mercury from industrial discharges.
Constructed wetland technology represents a possible cost-effective means of removing low-level mercury compared to more expensive treatment options such as ion-exchange. In wetland treatment systems, heavy metals such as lead, copper, arsenic, cadmium, and mercury are sorbed by organic compounds, clays, and other particles which increase residence time in sediments and hinder metal resuspension into the overlying water. Sulfur-based compounds derived from plant decomposition, plant exudates, or manual application also contribute to the metal binding capacity of freshwater wetland sediments. In anoxic environments, the reduced form of sulfur, sulfide (S2-) represents an important mechanism for metals precipitation (Dvorak, 1992; Barnett et al., 1997; Frandsen, 1999).
Sulfate-reducing bacteria (SRB) mediate the formation of sulfide as a result of respiration processes that require sulfate (SO42-) as a terminal electron acceptor (Brock et al., 1992). Dvorak et al. (1992) have examined the use of SRB in the treatment of metal-contaminated water. Others have also advocated a need for sulfate-reduction in the removal of metals from acid mine drainage (Hedin et al., 1989; Herlihy and Mills, 1985). However, the benefits of precipitating mercury as a sulfide complex using sulfate-amended sediments have not been fully characterized.
An obvious concern in using constructed wetlands to remove mercury is the potential formation of methylmercury (CH3Hg) since wetlands are known contributors of CH3Hg to downstream lakes and waters (St Louis et al., 1994; Hurley et al., 1995; Branfireum et al., 1998; 1999). Methylmercury is a lipophilic, organic form of mercury that is highly toxic and readily accumulated by aquatic organisms. Moreover, methylmercury is the fraction of total mercury most efficiently transferred up the food chain to higher trophic levels (Westcott and Kalff, 1996), and constitutes virtually all of the mercury measured in fish tissue (Bloom, 1992).
Previous studies in freshwater and marine systems have demonstrated that sulfate-reducing bacteria (SRB) are the major biological contributors of methylmercury formation in sediments (Anderson et al., 1990; King et al., 1999; King et al., 2000; Blum and Bartha, 1980; Devereux et al., 1996; Gilmour and Capone, 1987; Gilmour and Henry, 1991; Gilmour et al., 1992; Gilmour and Riedel, 1995; Gilmour et al., 1998). The respiration activity of SRB has been implicated in the methylation process. Molybdate, a specific inhibitor of sulfate reduction, has been used on numerous occasions to confirm that inhibition of sulfate reduction results in decreased methylmercury formation (King et al., 1999; Compeau and Bartha, 1985; Gilmour et al., 1992). Additional experiments have shown that SRB grown in pure culture do not methylate mercury in the absence of sulfate (King et al., 2000; Pak and Bartha, 1998). Moreover, a kinetic relationship relating sulfate reduction to mercury methylation has been documented providing further evidence that a "coupling" exists between the two processes (King et al., 1999; King et al., submitted). However, it should be noted that SRB, Desulfobulbus. proprionicus 1pr3 has demonstrated an ability to methylate mercury in pure culture under fermentative conditions (Benoit et al., 2001).
Early studies that identified SRB as mediators of biological methylmercury formation utilized Desulfovibrio desulfuricans as the species representative (Choi et al., 1994; Compeau and Bartha, 1985; Gilmour et al., 1991; Pak and Bartha, 1998). However, it is presumptuous to assume all SRB would methylate mercury at the same rate, since more than 19 genera of SRB have been described (Rooney-Varga et al., 1997). Recent literature suggests that the incidence of methylmercury formation, when normalized to sulfate reduction, is not uniform for all species of SRB (King et al., 2000). Experiments have shown that SRB groups capable of acetate utilization (e.g., Desulfobacteriaceae family) have a higher propensity for methylmercury formation than SRB that are unable to metabolize acetate (e.g., Desulfovibrionaceae family) (King et al., 2000). This characteristic has considerable importance since environments such as constructed wetlands may be more conducive to one consortia of SRB than others, depending upon the sediment composition, microbial interactions, and macrophyte contributions.
If constructed wetlands are to be mended with sulfate to enhance metals removal through sulfide-mediated precipitation, efforts should be made to identify parameters which optimize this process while minimizing methylmercury formation. As mentioned previously, the rate of sulfate reduction and consortia of SRB present in sediments have been identified as two such parameters which impact rates of methylmercury formation (King et al., 2000). A third parameter currently receiving considerable attention is the availability of mercury for microbial methylation. The complexation of sulfide with Hg2+ to produce insoluble cinnabar (HgS) has been described as a principal mechanism of decreasing mercury availability for methylation in aquatic systems (Gilmour et al., 1998; Gilmour and Capone, 1987; Anderson et al., 1990; Choi and Bartha, 1994). However, several studies have demonstrated the methylation of mercury in environments containing millimolar concentrations of sulfate and sulfide (King et al., 1999; King et al., submitted; J.E. Kostka, M.A. Frischer, and K. Maruya, unpublished results). Benoit et al. (1999 a & b) hypothesized that the availability of Hg for methylation is a function of neutral dissolved mercury complexes.
In this study, we initially characterized mercury removal efficiencies and methylmercury formation for a one-year period in gypsum-amended sediments planted with Scirpus californicus. In the final phase of the experiment, a submerged macrophyte, Potamogeton pusillus, was allowed to grow in one set of tanks and subsequent effects on SRB activity, SRB consortia profiles, and mercury binding ascertained.
Materials and Methods
Wetland treatment cell design
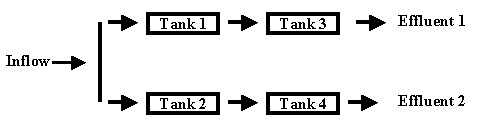
The experiment was devised such that wetland treatment cells (WTC) were exposed to outfall waters using a flow-through system (Figure 1). Tanks 1 and 2 received inflow directly from the outfall stream; tanks 3 and 4 received effluent discharge from tanks 1 and 2, respectively. Each WTC consisted of 2.45 x 0.6 x 0.9 m butyl rubber-lined plywood boxes. Tanks were filled with soils indigenous to the Savannah River flood plain and amended with time release fertilizer (58.0 kg-m-2), lime (68.4 kg-m-2) and organic matter (wood mulch: 3% by volume) to a depth of approximately 45 cm. Water was pumped from the waste stream into the first (upper) set of tanks (tanks 1 and 2) with release near the bottom of the tank. Discharge is through a slotted pipe set at a nominal elevation of 30 cm above the sediment surface, thus maintaining a 30 cm water depth in each of the tanks. Discharge from the upper tier of tanks flowed into the second (lower) tier of tanks (tanks 3 and 4) with input and output having the same configuration as the upper tanks (Figure 1). Nominal residence time in each tank is 24 hr with a design residence time for water passing through the first and second pair of tanks of 48 hr.
In late June 1999, the WTC were replanted with young Scirpus californicus plants that had been cloned from tissue cultures. Prior to planting the young plants, the water level in the wetland cells was drawn down to 2 inches. After planting the water level was raised by 2 inches per week until the target depth of 12 inches (30 cm) was reached. Three months after planting, Gypsum (CaSO4) was added to the surface of the soil at an application rate of 0.5% sulfur (by weight), based on the estimated mass of the top 10 cm of soil.
The system was allowed to operate for a period of eight months prior to an examination of the relationship between mercury speciation and the biogeochemical cycling of sulfur in the sediment. After eight months of operation, tanks 1 and 3 received additional organic matter in the form of decomposing Scirpus sp. (1 kg-m-2) which was applied at the surface/water interface. Tanks 2 and 4 did not receive additional organic matter. However, growth of a submerged macrophyte, Potamogeton pusillus, was permitted in tanks 2 and 4 while P. pusillus remained absent in tanks 1 and 3.
Sample Collection
Surface water total mercury and methylmercury samples were collected from each tank at the point of discharge for a period of six months beginning in May 1999. Both filtered and unfiltered surface water samples were collected. Filtered samples were collected in hydrochloric acid-washed, polypropylene syringes and filtered through 0.45 m m cellulose acetate syringe filters (Gelman scientific, Ann Arbor, MI). Porewater total mercury and methylmercury samples were collected during the same time period using "sippers" as described by Short et al. (1985). Sippers were deployed such that single samples were collected over 3 cm depth intervals which included: 0-3 cm, 3-6 cm, 6-9 cm, and 9-12 cm. Two sippers were deployed at the same depth interval per tank, and sample fractions from each sipper were pooled in acid-washed, polypropylene syringes then filtered as described previously. Porewater samples were also collected and pooled to determine sulfide concentrations. Data acquired from the 0-6 cm depth was averaged and reported as a single value. The same method was used for data collected at depths ranging from 6-12 cm.
Sulfate-reduction rates (SRR) were quantified using cores (10 cm length) taken in duplicate from tanks 1 and 2 in September 2000.
Sediments used for SRB identification were also collected from sediment cores in September 2000. Duplicate cores (10 cm length) were taken from tanks 1 and 2 and sectioned into 3 cm intervals. Duplicate cores from the same tank were homogenized prior to cell isolation.
Sample Analysis
Water samples were analyzed by the Skidaway Institute of Oceanography for total mercury and methylmercury. All samples were collected using "Ultra-Clean Collection of Water and Wastewater Samples for the Analysis of Low Level Mercury" (a modified version of EPA method 1669), also referred to as the "clean hands-dirty hands" technique (Bloom, 1993). Total mercury was quantitated in water samples using EPA low-level detection method 1631 (USEPA, 1999; Liang and Bloom, 1993). Methylmercury was quantitated using EPA low-level detection method 1630 (Bloom and Von Der Geest, 1995; Liang et al., 1994).
SRR was determined by using a radiolabled tracer technique developed by Jørgensen (1978) and a one-step sulfide distillation procedure performed as described by Fossing and Jørgensen (1989). Sediment cores were injected with 2 m l of 35SO42- (2.2 mCi ml-1; ICN Biomedicals Inc.) at 1 cm intervals. The core was allowed to incubate for a period of 6 hours at 25°C after which it was sectioned into 3 cm depth intervals, homogenized, and analyzed. Sulfate concentrations required in SRR calculation were determined using an ion chromatograph (Dionex DX-100 with IonPac As4A 4mm column).
Sulfide concentrations were measured by colorimetric analysis (Cline, 1969).
Bacterial cells were isolated from sediments using methods previously described by Frischer et al. (2000). The oligonucleotide probes were synthesized at the molecular genetics facility of the University of Georgia by using an ABI model 394 DNA-RNA synthesizer. Labeling and probing of cells was determined using methods described by Devereux et al. (1992). Protocol for use of probes is reported in King et al. (2000). In brief, a targeted 32P labeled probe was added to blots that were contained in 50 mL hybridization tubes (Bockel Scientific, Feasterville, PA). Tubes containing the blots were incubated in a rotary hybridization oven (Robbins Scientific Model 2000 microhybridization incubator, Robbins Scientific, Corp., Sunnvale, CA) for 18 h. Blots were then washed and exposed to film (Fuji Medical X-Ray film, 20.3 x 25.4 cm, Fuji Medical Systems U.S.A.) in autoradiograph cassettes (FBXC 810, Fisher Scientific) with enhancement screen at –80°C. The specificity of probes and relative percent abundance of sulfate-reducing bacteria was determined using a densitometer (420 OE, PDI Inc.).
Results
Results compiled over one year demonstrate that mercury was consistently removed from outfall waters passing through the mesocosm cells (Table 1). Total mercury concentrations in outfall waters used as influent to cells varied with each monthly sampling. Overall, removal efficiencies for both tanks averaged approximately 50 % for the first year of operation. Five samples collected from the waste stream had total mercury concentrations that exceeded 200 ng-l-1 and were thought to be spikes in concentrations derived from facility discharges rather than sustained concentrations. As a result, removal efficiencies were only considered for outfall total mercury concentrations less than 200 ng-l-1 when reporting the average.
Methylmercury concentrations were collected over the same time period as total mercury. Overall, inflow concentrations of methylmercury were typically less than the limit of detection (< 0.02 ng-l-1). Initial concentrations of methylmercury in the effluent ranged from 0.081 to 0.241 ng-l-1 for the first three months of operation (Figure 2). However, methylmercury concentrations exceeded 1.0 ng-l-1 for both mesocosm trains after surface applications of gypsum (CaSO4) in September 1999 (Figure 2). In general, surface water concentrations of methylmercury then decreased in mesocosm trains from November 1999 to Feburary 2000. This was followed by a trend towards increasing methylmercury concentrations starting in March 2000 with maximum methylmercury concentrations detected at the end of May 2000 (Figure 2).
Filtered and unfiltered total mercury samples were collected for each tank beginning in June 2000. The percentage of mercury able to pass through a 0.45 m m filter varied considerably throughout the summer (Table 2). Overall, the percentage of mercury recovered after filtration was considerably lower in the tank 2 (Potamogeton sp. inhabited) as compared to tank 1. Excluding July, the percentage of total mercury that could pass through a 0.45 m m filter in tank 4 (Potamogeton sp. inhabited) was considerably lower than the amount in tank 3 (Table 2).
Porewater profiles of total mercury and methylmercury were analyzed in the two mesocosm trains. Overall, concentrations of methylmercury in porewater decreased from May to July 2000 (Figure 3). However, a spike in methylmercury concentrations was observed in all tanks in August 2000. Total mercury in porewater followed similar trends and is reported in Figure 4.
Sulfide concentrations were at or below detection limits in June and July of 2000. However, increases in porewater sulfide were observed in all tanks in August (Figure 5). Levels of sulfide in porewaters subsequently decreased by September (Figure 5).
Sulfate-reduction rates (SRR) were quantified in tanks 1 and 2 during the month of August using 35S-radiolabled sulfate. Overall, the average rates of sulfate reduction in the upper 9 cm were higher in tank 1 than tank 2 (Figure 6). Moreover, significant variations in vertical SRR profiles existed between the two tanks. SRR in tank 1 were maximum at the surface/water interface and the 6-9 cm depth while the SRR in tank 2 had an average maximum rate at the 3-6 cm depth.
Five groups of sulfate-reducing bacteria (SRB) were identified in sediment cores taken from tanks 1 and 2 (Figure 7). The relative abundance for all SRB (expressed in terms of the universal probe) indicate that a subsurface maximum exists for tank 2 while maximum relative abundance measurements for tank 1 were comparable between 0-3 and 3-6 cm (Figure 7). In tank 1, Desulfobacter sp. had a relative abundance greater than 75% to a depth of 9 cm. This differed dramatically from tank 2 where relative abundance averaged less than 30% for Desulfobacter sp. at depths greater than 3 cm. Maximum relative abundance for Desulfovibrio, Desulfobulbus Desulfococcus, and Desulfobacterium groups occurred in the 9-12 cm range for tank 1. Desulfobulbus and Desulfococcus sp. groups were also observed at greater than 75% abundance in the upper 0-3 cm of tank 1 (Figure 7). Excluding Desulfobacter sp., all groups in tank 2 increased with respect to relative abundance measurements as depth increased.
Discussion
The gypsum-amended, constructed WTC demonstrated a capability to remove low-level mercury (ng-l-1) from outfall streams. However, additions of sulfate to the cells resulted in 8-fold increases in methylmercury production. Increased rates of methylmercury formation have been demonstrated in sediment systems amended with sulfate (Branfireum et al., 1999; Gilmour et al., 1992). These enhanced MMR resulting from sulfate addition have been attributed to increases in SRB activity and population density (Branfireum et al., 1999). Following CaSO4 addition, rates of methylmercury formation in the mesocosm planted with Scirpus californicus followed a seasonal cycle with respect to observed maximum and minimum methylmercury concentrations (Figure 2). These results are similar to those documented in other sediment-based systems where concentrations of methylmercury and mercury methylation rates were recorded over time (Hines et al., 1989; Korthals and Winfrey, 1987). Higher methylmercury concentrations in late spring and early summer are attributed to increased SRB activity (i.e., increased SRR). Increased SRR in sediments planted with Spartina alterniflora and Ulva rigida have also been documented in May and June during the aboveground growth phase of respective plant species (Gianmarco et al., 1997; Hines et al., 1989). Scirpus aboveground growth has been documented during these months (Huddleston et al., submitted). Warmer temperatures combined with release of exudates from initial growth phase of plants have been demonstrated to stimulate SRB activity (Hines et al., 1989). Since MMR demonstrated a unique coupling to SRB activity (i.e., SRR) in the presence of "available" mercury, increased SRB activity in May is suggested here as the reason for observed increases in methylmercury concentrations during late spring/early summer (King et al., 1999). Flowering of Scirpus plants occurred in late July corresponding to a decrease in observed methylmercury concentrations in porewater and surface water effluents (Figures 3 and 4). Hines et al. (1989) reported that the flowering of Spartina plants resulted in the rapid reallocation of carbon to reproductive organs resulting in a four-fold decrease in sediment SRR. Moreover, after flowering occurs carbohydrates are prevented from exuding through roots as a result of immobilization in rhizomes (Lytle and Hull, 1980). The frequency with which organic acids were observed in mesocosm sediments decreased after flowering of Scirpus plants (data not shown). Presumably a similar decrease in SRR, as reported by Hines et al. (1989), occurred as a result of diminished substrate availability. Thus, methylmercury formation mediated by SRB decreased after Scirpus flowering.
Sulfide concentrations in gypsum-amended tanks were at or near the method limit of detection for the months of June and July. However, substantial increases in porewater sulfides were observed in all tanks beginning in August (Figure 5). Values reported for June and July suggest that the rates of sulfide removal from porewaters were faster prior to Scirpus flowering (late July) with a buildup of sulfide occurring afterwards. Studies have reported that sediment oxidation does occur as a result of plant-mediated evapotranspiration and exudate release, and these processes are most active during the aboveground growth phase prior to flowering (Dacey and Howes, 1984; Howes et al., 1986). Our results are similar to those reported by Hines et al. (1989) which demonstrated increased sulfide concentrations in porewaters after S. alterniflora flowering. In our study, concentrations of sulfide increased immediately after flowering of S. californicus (Figure 5). A decrease in porewater sulfide concentrations followed the mid-September maximum. This may reflect a decrease in SRR and continued metal-sulfide precipitation that occurred with outfall metals: Zn, Hg, and Cu.
It is interesting to note that the increase in porewater sulfide (Figure 5) corresponded to an increase in porewater methylmercury and total mercury concentrations (Figures 3 and 4). Faust and Osman (1981) have reported on the binding of sulfide with Hg2+ to form an insoluble HgS. While equilibrium constraints indicate that a large fraction of mercury would be bound up as a precipitate in a sulfide-rich environment, this does not preclude the existence of soluble Hg-sulfide species that are also subject to equilibrium constraints. Paquette and Helz (1997) have demonstrated that soluble Hg2+ concentrations increase in the presence of zero-valent sulfur (S0), a constituent of porewater-reduced sulfur. In addition, Benoit et al. (1999a) also found a positive correlation between dissolved Hg2+ and porewater sulfide in the Patuxent River Estuary. Availability of mercury for methylation has been attributed to the presence of neutral dissolved mercury complexes such as HgS0 (Benoit et al., 1999a&b). Conceivably elevations in porewater sulfide (an increase from non-detectable levels) resulted in an increased fraction of neutral dissolved mercury that was available for methylation. This explanation seems plausible given the simultaneous increase in dissolved inorganic mercury and micro-molar increase in porewater sulfide. It should be noted that neutral dissolved HgS0 complexes decrease as sulfide concentrations approach 10-3 M where HgHS2- has been reported as the dominant soluble mercury species (Benoit et al., 1999b). Thus, it is expected that increases in porewater methylmercury concentrations would not be as pronounced in environments where sulfide concentrations increased above micro-molar levels.
The effect of submerged macrophytes on mercury removal in constructed wetlands was also considered. In addition to Scirpus californicus, Potamageton pusillus was allowed to grow in tanks 2 and 4 beginning in June 2000. The presence of the submerged macrophyte, P. pusillus, resulted in a greater amounts of visible suspended particulate matter, as well as a greater concentration of total mercury remaining in the unfiltered, overlying waters of tank 2 as compared to tank 1 (Table 2). Filter studies also demonstrate that a greater percentage of total mercury is bound to SPM in tanks containing P. pusillus and thus, not recovered after filtration (Table 2). A relationship between SPM and mercury distribution in water has been explained both in freshwater and estuarine systems (Coquery et al., 1995; Cai et al., 1999). However, the relationship between Potamogeton sp. and the observed relative increase in SPM is not clear.
In late August, measurements of SRB community structure and activity also differed between the two mesocosms. In tank 1, SRB phylogenetic groups Desulfobulbus, Desulfococcus, and Desulfobacter had maximum or near maximum relative abundance values in the upper 3 cm of the sediment cores (Figure 7). A possible explanation for this observation might be the additional organic matter loaded on the sediment surface. Conceivably, the decaying Scirpus material amended to the surface of the sediments would supply an additional carbon source that would support increased bacterial colonization. Moreover, a result of having a 2-3 cm thick layer of organic-rich material above the sediment would result in a reduction of redox potentials in sediments located below the organic matter (i.e., decaying Scirpus). This effect would also serve to stimulate colonization of anaerobic SRB into regions of the sediment that would otherwise be susceptible to fluctuations in redox conditions.
Surface water methylmercury concentrations and flux of methylmercury out of the system increased in the month of August in cells treated with additional organic matter (Figure 2). However, cells with P. pusillus did not generate a similar response (Figure 2). The reasons for the differences in observations are unclear. The relative abundance of Desulfobacter sp. remained at high levels in tank 1 to a depth of 9 cm compared to only a surface maximum in tank 2 (Figure 7). The Desulfobacteriaceae family, which includes Desulfobacter sp. has been identified as having the greatest propensity for mercury methylation when compared to members of the Desulfovibrioaceae family (King et al., 2000; Rooney-Varga et al., 1998). Although mercury methylation rates (MMR) were not established in these systems, it is interesting to note that the flux of methylmercury from tank 1 (large abundance of Desulfobacter sp.) increased in the month of August compared to no observable increase in tank 2 (Figure 2). Sulfate-reduction rates (SRR) have also been identified as contributing to rates of mercury methylation (King et al., 1999; King et al. submitted). SRR profiles for tank 1 were higher than those reported for tank 2 for all depths excluding 9-12 cm (Figure 6). Higher rates of sulfate-reduction could also provide for increased MMR and thus a possible explanation for the higher flux of mercury out of tank 1 in August. The "availability" of mercury for methylation represents a third component that determines rates of methylmercury formation. In these mesocosm systems, the amount of total mercury in tanks 2 and 4 that was present in the dissolved phase as defined by Cai al. (1999) was less than that observed in tank 1 and 3, respectively for all sampling periods including August (Table 2). It is widely accepted that sulfur chemistry plays a dominant role in mercury speciation and availability in the dissolved phase (Benoit et al., 1999a&b; Paquette and Helz; 1997). However, very little is known about mercury availability as it relates to complexation with SPM. Benoit et al. (1999b) suggested that the most favorable form of mercury for methylation is an uncharged mercury sulfide complex that can freely pass through cell membranes. Given the size of SPM (>0.45 m m), mercury complexed with SPM would not be available for immediate transport into cells and thus would retard rates of bacterial methylation. Results from our WTC appear to support this hypothesis.
Since no increase in methylmercury was observed in surface waters of tank 2 and 4 during the month of August, the influence Potamogeton pusillus has on demethylation and microbial consortia profiles at the sediment/water interface must also be considered. Roots of P. pusillus found exclusively in the upper 3-4 cm of sediments in tanks 2 and 4 could supply subsurface oxidants including oxygen via root exudates. The presence of oxidants has been attributed to increased rates of demethylation while inhibiting methylation (Korthals and Winfrey, 1987; Compeau and Bartha, 1984). In tank 2, larger relative abundance values for SRB phylogenetic profiles of Desulfovibiro, Desulfobulbus, Desulfococcus, and Desulfobacterium were located at deeper depths also suggesting possible oxidative influences in upper centimeters of the sediment (Figure 7). These findings suggest more favorable growth conditions exist at deeper depths for anaerobic bacteria in tank 2.
Overall, the influence of the Scirpus californicus lifecycle on sulfur chemistry in gypsum-amended, freshwater sediments was similar to Spartina alterniflora in marine environments. It should be noted that sulfide concentrations in marine systems reach concentrations that are orders of magnitude higher than those observed in our study (Hines et al., 1989; J.E. Kostka, unpublished results). Therefore, the sulfur cycle or metal binding potentials observed in estuarine environments cannot be used to predict metals removal or methylmercury formation in constructed wetlands that have been amended with sulfate. Future studies must focus on the use of constructed wetland technology and how additions of a sulfur source such as gypsum affect freshwater, biogeochemical processes and metal removal. Studies using constructed wetlands to remove parts-per-trillion mercury levels should also ascertain what influence, if any, Potamogeton species have on demethylation potentials at the sediment/water interface and the possibility of decreasing concentrations of mercury that are "available" for methylation using Potamogeton-derived SPM.
Acknowledgments
The authors gratefully acknowledge Ralph Smith, Debbie Wells, and Lori Cowden for their expert technical assistance.
References

Figure 1. Diagram of wetland treatment cell design.
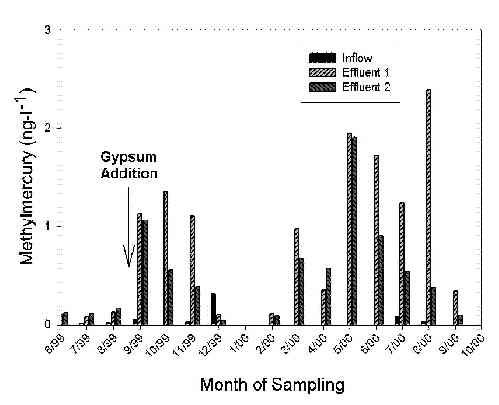
Figure 2. Methylmercury concentrations found in respective effluent
streams.
Gypsum (CaSO4) added prior to September 1999 sampling.
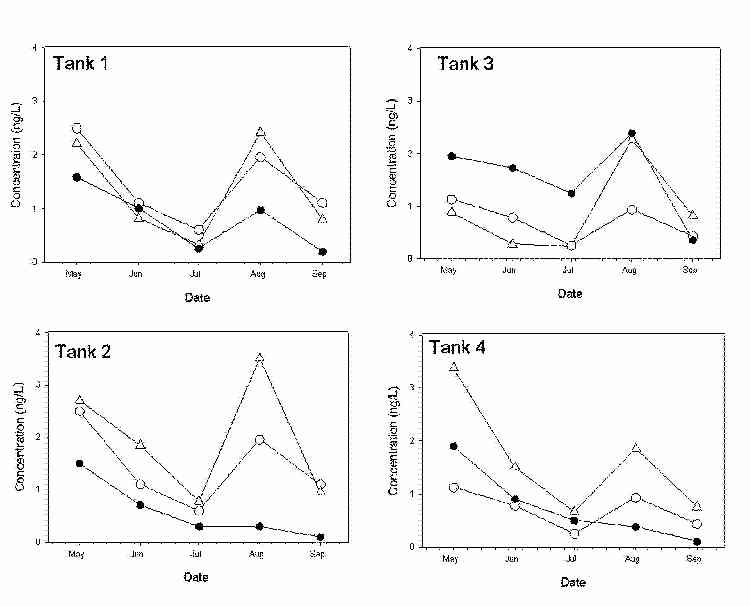
Figure 3. Methylmercury concentrations in surface water (●) and in porewater at depths of 0-6 cm (∆), and 6-12 cm (○).
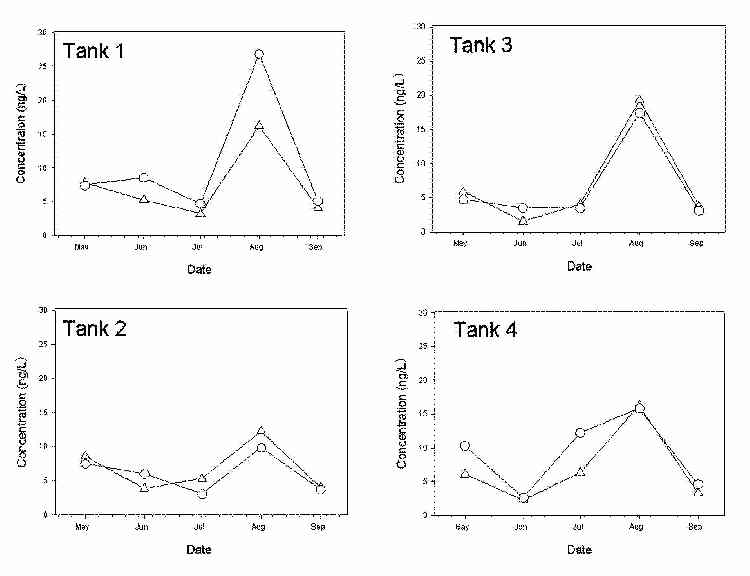
Figure 4. Total mercury concentrations in porewater at depths of 0-6 cm (∆), and 6-12 cm (○).
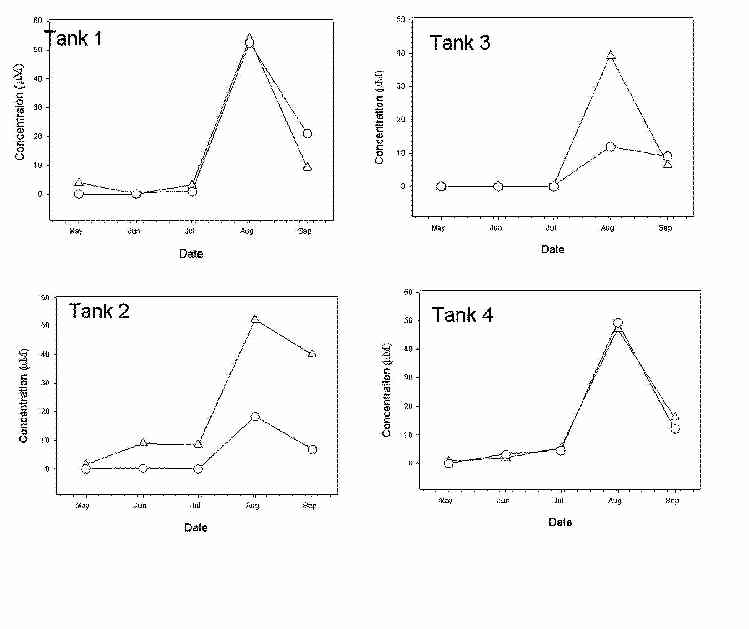
Figure 5. Porewater sulfide concentrations at depths of 0-6 cm (∆) and 6-12 cm (○).
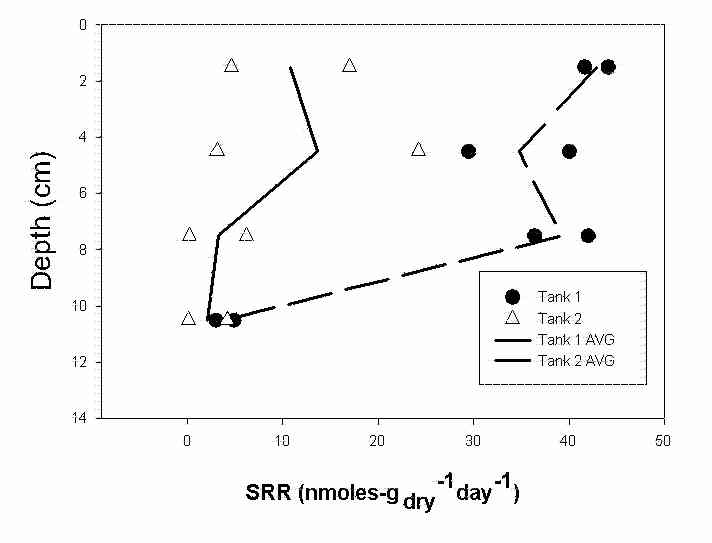
Figure 6. Sulfate-reduction rates acquired during the month of August 2000 for tanks 1 and 2.
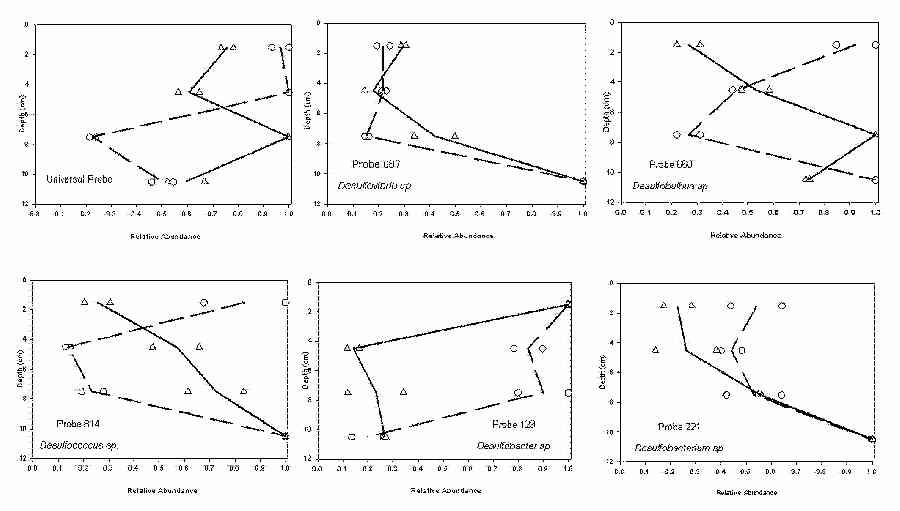
Figure 7. 16S rRNA relative abundance profiles for sulfate-reducing bacteria in tank 1 (○) and tank 2 (∆).