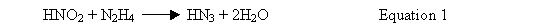
WSRC-TR-2000-00443
Hydrazoic Acid Controls and Risks When Processing
Plutonium Solutions in HB-Line Phase II
D. F. Hallman
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
1. Summary
The concentration of hydrazoic acid can be controlled below the explosive limit in both liquid and vapor by controlling the maximum concentration of hydrazine mononitrate in the acid solution. The hydrazine concentration will be controlled at or below 0.15 M in the plutonium solutions to maintain the hydrazoic acid concentration below explosive limits. This form of control has been successfully used in HB-Line over many years, most recently during the Cassini campaign. Implementation of the controls specified in this report result in a frequency of Beyond Extremely Unlikely (BEU) for an explosion in either the liquid or vapor phase of the process vessels.
The offsite consequences of a hydrazoic acid explosion is bounded by the Low Assay Plutonium hydrogen explosion.
Keywords: Explosion, Hydrazoic Acid, AB Controls
2. Introduction
The feed solution for plutonium processing in HB-Line Phase II is relatively dilute (6.75-grams/liter maximum assumed for consequence calculations) plutonium in nitric acid solution. There are two tanks in H-Canyon that have collected plutonium streams over the years, and more solution is being generated as plutonium-bearing scrap is being dissolved in HB-Line Phase II and stored in H-Canyon tanks.
Phase II contains two parallel trains for processing plutonium solutions. Generally, the trains are operated independently. Source terms are calculated based on simultaneous operation of both trains, but this is not planned. Where two equipment designators are used below in parentheses, they refer to equipment in both trains.
For plutonium processing in HB-Line, hydrazine is not used before the concentrated plutonium stream is eluted from the ion exchange columns. This solution, containing high (~ 30 grams/liter plutonium) plutonium concentrations is collected in the concentrate tanks (NT-21 and NT-22). Batches of the concentrated solution, containing about 1000 to 1250 grams of plutonium, are then transferred to the feed adjust tanks (NT-41 and NT-42). In these tanks, a liquid solution of hydrazine mononitrate is added to scavenge the small amount of nitrous acid impurity expected to be found in a nitric acid solution. Hydrazine mononitrite reacts with nitrous acid to form hydrazoic acid. Then, an ascorbic acid solution is added to adjust the plutonium to the + 3 valence state.
The plutonium solution will then be pumped to the precipitation tanks for precipitation with oxalic acid to form solid plutonium oxalate. The process of adding the solution to be precipitated (plutonium nitrate) to the precipitating solution (oxalic acid) is called the "reverse strike" method. There is a small nominally four liter first stage precipitation tank (NP-1 and NP-3) that sits on top of a nominally 75 liter second stage precipitator (NP-2 and NP-4). This gives the capability to perform simultaneous feed, two stage precipitation. However, for the reverse strike method, no mixing occurs in this first stage tank, and the valve on the bottom of the tank is fully opened to allow flow into the second stage tank.
Any hydrazoic acid formed in the first stage tank flows into the precipitator tank.
The solution in the precipitator tank is stirred until precipitation is complete. This solution, now containing plutonium oxalate solids, excess oxalic acid, and any hydrazoic acid formed, is then vacuum transferred through a filter for solids separation. The liquid flows through the filter into a nominally 100-liter filtrate tank (NT-51 and NT-52). Any hydrazoic acid formed is transported with the liquid into the filtrate tank. About 5 liters of a weak (about 2.4 molar) nitric acid stream is used to wash the cake, and this liquid also goes to the filtrate tanks.
Sodium permanganate is then added to the filtrate tank solution. This is added to decompose the oxalic acid excess used to ensure complete precipitation of the plutonium nitrate. It also decomposes the oxalate associated with any solid plutonium oxalate that passes through the filter. This is necessary to prevent solid plutonium oxalate formation in any tank the solution is educted to (typically an H-Canyon waste tank) and potential criticality from accumulation of plutonium solids. The sodium permanganate also oxidizes any hydrazoic acid that is present, and any hydrazine residual that is present. Sodium nitrite is then added, if necessary, to dissolve any manganese dioxide formed from the sodium permanganate addition. Since any hydrazine residual had been destroyed by the permanganate, no hydrazoic acid is formed from the nitrite addition.
3. Methods of Analysis
The method of formation of hydrazoic acid while processing plutonium solutions in Phase II of HB-Line is considered in deriving controls to prevent (decrease the frequency of) a hydrazoic acid explosion. The frequency of a hydrazoic acid explosion is evaluated considering the failures required to reach conditions where an explosion can potentially occur and the credible locations of a hydrazoic acid explosion. The controls that reduce the consequences and frequency of a hydrazoic acid explosion in HB-Line for processing plutonium solutions are identified in the individual evaluations, then summarized in the Assumptions section to enhance ease of identification of the controls.
A quantitative comparison of source terms is used to demonstrate that the consequences of a hydrazoic acid explosion is bounded by the previously-calculated consequences of a hydrogen explosion while processing Low Assay Plutonium (LAP). Both source terms are reduced to the equivalent mass of a reference isotope for this comparison
4. Results
Background information on the formation of hydrazoic acid, and the mechanism for formation of an explosive mixture, is discussed further below, to establish the basis for the controls selected to prevent a hydrazoic acid explosion. The methods used to evaluate the frequency and consequences of a hydrazoic acid explosion in Phase II of HB-Line are also discussed below. Also, the controls used to prevent a hydrazoic acid explosion are discussed.
4.1 Background
The reasons for hydrazine mononitrate usage as a chemical additive are discussed above. Hydrazine mononitrate requires special handling during addition to minimize personnel exposure, but this is a common chemical hazard and is controlled through the WSRC Chemical Control Program (Reference 1) and will not be discussed further in this Technical Report. Hydrazine mononitrate is not a processing hazard except for its contribution to hydrazoic acid formation.
Hydrazine mononitrate reacts with nitrous acid (HNO2) to produce two molecules of water and a molecule of hydrazoic acid (HN3), as shown in Equation 1 (Reference 2).
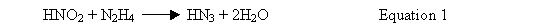
Hydrazoic acid also reacts with nitrous acid to produce water and gaseous products, as shown in Equation 2.
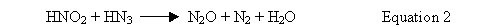
The equilibrium amount of hydrazoic acid in solution depends on the relative rates of Equations 1 and 2. Laboratory experiments show that, because of the reaction of hydrazoic acid with nitrous acid (Equation 2 above) the equilibrium concentration of hydrazoic acid in liquid is about 25% of the hydrazine concentration. At higher hydrazine concentrations (0.19M and 0.44M) the maximum hydrazoic acid concentration in the liquid was 12% of the hydrazine concentration (Reference 3). In this evaluation, it is conservatively assumed the hydrazoic acid concentration can temporarily be as large as 1/3 the hydrazine mononitrate value. Therefore, if the hydrazine concentration in solution is less than or equal to 0.15M, the hydrazoic acid concentration will be less than 0.05M.
It is reported (Reference 4) that solutions must contain at least 30 weight % (~ 8.4M) hydrazoic acid before an explosion can occur in liquid. Therefore, if the concentration of hydrazine used for nitrous acid scavenging is limited below (8.4*3 ~ 25.2M) an explosive mixture in liquid cannot form.
4.2 Frequency of a hydrazoic acid explosion in the liquid phase
HB-Line uses a nominally 30 weight % hydrazine mononitrate [N2H4× HNO3] solution in water for valence adjustment. Assume a bounding hydrazine concentration in the feed solution is 35 weight % to ensure a bounding value of hydrazine concentration. Conservatively assuming a solution density of 1.021 grams/cc (the density of pure hydrazine), the weight of hydrazine mononitrate per liter is 306.3 grams, in a 35% solution, the hydrazine mononitrate weight would be 357.35 grams/liter. Hydrazine mononitrate has a molecular weight of 95 grams/mole, so the molarity of hydrazine mononitrate in a 35 weight % solution is 3.76M. This molarity cannot be practically attained in solution, since the hydrazine mononitrate is diluted by the solution it is added to. This is far below the limit of 25.4M hydrazine at which an explosive concentration of hydrazoic acid in liquid might occur. Therefore, by limiting the hydrazine supply to be added for nitrous acid scavenging to less than 35 weight % hydrazine mononitrate, HB-Line stays about a factor of six below the hydrazine molarity that could cause explosive concentrations of hydrazoic acid in the liquid, even with uncontrolled addition.
If pure hydrazine mononitrate were used for feed adjustment, the molarity would be ~ 10.75M, using the methods discussed above. There are many reasons, including potential chemical exposure to personnel, that this strength hydrazine mononitrate would not be acceptable to use as a feed. Also, the two drums of ~ 30 weight % hydrazine mononitrate are sufficient for the Phase II processing of Pu that is now scheduled. However, assume errors by the vendor resulted in material being shipped to WSRC, and errors in the chemical control program, this concentration of hydrazine mononitrate is still below the limit of 25.2M that could result in 8.4M hydrazoic acid in solution. Therefore, the frequency of achieving an 8.4M hydrazoic acid concentration in the liquid phase is. Beyond Extremely Unlikely (BEU).
Hydrazoic acid can also concentrate in condensate if the condensing temperature is lower than the evaporation temperature. In HB-Line Phase II, the pertinent condensation point would be the vessel vent system. The vessel vent system is connected to all the Phase II vessels that contain process solutions, and a vacuum is maintained on this system to draw vapors from the vessels for cleanup before discharging the vessel vent system. The concentration of hydrazoic acid in the vapor phase increases as the solution temperature increases, and the concentration in the condensed liquid increases as the condensation temperature is lowered. The hydrazoic acid concentration in condensate in Phase II of HB-Line was evaluated using extreme conditions of evaporation at 50 ° C, condensing at 0 ° C, and 0.15M hydrazoic acid in the liquid phase (Reference 4). The resulting concentration in the condensing vapor is 1.27M hydrazoic acid. This is also well below the explosive concentration of 8.4M. This concentration could only practically exist on the walls of vessel vent system piping prior to entry to the vent catch tank. This tank contains about 100 liters of water and would dilute the small amounts of hydrazoic acid transported through the vessel vent system. Further, there is no source term, other than trace levels of radioactivity, in the vessel vent system tubing and tanks, so the consequences of a liquid explosion would be very low. No additional controls will be implemented to control this small risk; controls that make the explosions in process vessel liquid and vapor BEU are adequate to control this small risk.
4.3 Frequency of a Hydrazoic Acid Explosion in the Vapor Phase
After filtration, the hydrazoic acid may be present in the filtrate tank. Sodium permanganate will be added to the filtrate tank to decompose the oxalate before transfer to H-Canyon. The sodium permanganate addition decomposes both the hydrazine and the hydrazoic acid, so the risk of a hydrazoic acid explosion ends when the permanganate is added to the filtrate tank. Reaction kinetics favor the oxidation of hydrazoic acid and hydrazine over the oxidation of oxalate by the permanganate solution. No hydrazoic acid will be present in solutions downstream of the filtrate tanks.
Hydrazine is stored in drums (maximum two) on the sixth level of HB-Line in an area with special drainage and ventilation. An individual must take a container to a drum, fill the container to the proper amount at the drum, transport the container over to a funnel valve leading to the feed adjust tank, open the funnel valve, add the hydrazine through this valve, then close the valve after hydrazine addition. There is no piped path between the hydrazine source and the feed adjust tanks, so it is impossible to add hydrazine by valving errors or inadvertent leakage through valves or fittings.
The partial pressure of hydrazoic acid in the vapor phase where decomposition may occur (the first reaction in Table 5 in Section 4.4) is 65 torr (Reference 5). There are 760 torr in an atmosphere; therefore, the volume percent at which hydrazoic acid can decompose is (68/760) ~ 8.9%, or 0.089 atmospheres. The Henry’s Law coefficient of hydrazoic acid in water at 30 ° C is 0.1 atmospheres vapor/(moles/liter in nitric acid). This coefficient decreases as nitric acid concentration increases and as temperature decreases, so the use of 0.1 bounds the operating conditions. Using a bounding Henry’s Law coefficient bounds the vapor phase hydrazoic acid concentration. The amount of vapor phase hydrazoic acid that exists in equilibrium above a hydrazoic acid solution is given by the following equation:

The concentration of hydrazoic acid in liquid is conservatively assumed to be 1/3 of the hydrazine (N2H4) concentration. Table 1 shows the concentration of hydrazoic acid in the vapor phase as a result of hydrazine additions.
Table 1. Vapor phase hydrazoic acid concentration for hydrazine
additions, adding
constant volume each time of 30% hydrazine mononitrate
|
Number of |
Hydrazine |
Hydrazoic |
Hydrazoic |
Fraction of |
|
Hydrazine |
Concentration, |
Acid |
Acid |
Hydrazoic |
|
Adds |
in Liquid, |
Concentration |
Concentration |
Acid |
|
Moles/Liter |
In Liquid, |
in Vapor, |
Decomposition |
|
|
Moles/Liter |
Volume % |
Concentration |
||
|
in Vapor |
||||
|
1 |
0.050 |
0.017 |
0.167 |
0.019 |
|
2 |
0.098 |
0.033 |
0.328 |
0.038 |
|
3 |
0.145 |
0.048 |
0.485 |
0.057 |
|
5 |
0.235 |
0.078 |
0.785 |
0.092 |
|
10 |
0.439 |
0.146 |
1.462 |
0.171 |
|
20 |
0.772 |
0.257 |
2.574 |
0.301 |
|
30 |
1.034 |
0.345 |
3.448 |
0.403 |
|
40 |
1.246 |
0.415 |
4.152 |
0.485 |
|
50 |
1.420 |
0.473 |
4.733 |
0.553 |
|
70 |
1.690 |
0.563 |
5.632 |
0.659 |
|
100 |
1.971 |
0.657 |
6.569 |
0.768 |
The process requirement, and the amount specified to be added, for hydrazine concentration is 0.05M. This table shows that over 100 times the planned amount of hydrazine must be added to provide a hydrazoic acid vapor phase concentration that can undergo a decomposition reaction. The concentration of hydrazine in liquid that, if converted to hydrazoic acid at 1/3 the hydrazine concentration, would result in a vapor concentration subject to decomposition, is 2.67M.
It is not clear that the decomposition of hydrazoic acid in the vapor phase is a combustion reaction. NFPA 69 does not apply to non-combustion reactions. But, the control on hydrazine will maintain the hydrazoic acid concentration in the vapor phase at a maximum concentration of six percent of the decomposition concentration (see line 3 of Table 1 above). This is well within the NFPA controls for gaseous species that undergo combustion reactions (such as hydrogen) at less than or equal to 25% of the Lower Flammability Limit (LFL).
The control on hydrazine concentration includes several methods to ensure the control is properly implemented. These are as follows:
Reference 6 documents an evaluation of the frequency of adding enough hydrazine to form an explosive concentration of hydrazoic acid in vapor, and concludes this probability is 7.5E-10/run. Conservatively assuming six runs per week (three per line per week) and 52 operating weeks, the frequency of a hydrazoic acid vapor explosion is 2.34E-07/year. This frequency is BEU.
In addition to the above analysis, there would be insufficient nitrous acid to form the concentration of hydrazoic acid needed to have an explosive mixture in the vapor space. Nitrous acid is an impurity in nitric acid, and is also formed by reaction of ferrous sulfamate with nitric acid. Ferrous sulfamate is a treatment chemical for resin column feed solutions, to adjust the plutonium valence, but only small quantities would be formed here. Further, the feed solution is sent to waste after passing through the ion exchange column, and is not treated with hydrazine. However, no credit is taken in the above analysis for the limited amount of nitrous acid in the solutions treated with hydrazine.
4.4 Consequences of a hydrazoic acid explosion
For BEU events, no consequence calculations are required. A comparison of offsite consequences of a vapor phase explosion is presented here, in comparison to the consequences of a vapor phase explosion of hydrogen performed for the Low Assay Plutonium (LAP) campaign. Liquid phase explosions have not been credible events in previous HB-Line campaigns, so no comparisons can be made. Resources were not expended to perform an independent liquid phase explosion consequence calculation since the event is BEU.
The consequences to the public and the onsite worker of a vapor phase hydrazoic acid explosion while processing plutonium solutions in Phase II are bounded by the consequences of a hydrogen explosion when processing Low Assay Plutonium (LAP). These are reported in the HB-Line Basis for Interim Operation (BIO) (Reference 7). From Table ES-1 of this document, the consequences to the public are 0.2 Rem, and the consequences to the onsite worker are 1.9 Rem. Both of these results are well below the evaluation guidelines for consequences. The HB-Line building structure and the H-Canyon exhaust tunnel was credited with performing a confinement function during the hydrogen explosion, and the H-Canyon sand filter and exhaust fan were credited with filtering and transporting exhaust from HB-Line during the accident. The vessels containing radioactivity and the gloveboxes containing the vessels were not credited with mitigating the consequences.
DOE-HDBK-3010-94 (Reference 8) provides information on the fraction of radioactive sources released during energetic events. For explosions, the amount of material released is the amount of dynamite that would give an explosion of the same energy.
The source term used for the LAP hydrogen explosion is provided in S-CLC-H-00649, Rev. 0 (Reference 9), and is 2,988 grams. This material is assumed to be 85% Pu-238 and 15% Pu-240 by weight. The Pu-239 isotopic distribution is quite different; the maximum Pu-238 in this distribution is 1% by weight.
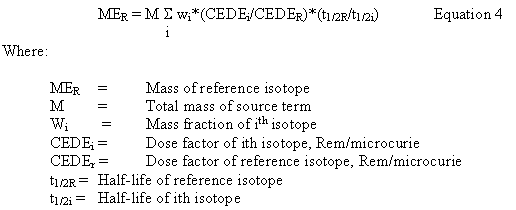
Two sources may be compared directly for their dose effect by reducing both to the gram equivalency of a reference isotope. Each isotope is converted to curies. Then, equivalent curies of another isotope are obtained by multiplying the curies of each isotope by the dose factor for that isotope, and dividing this result by the curies of the reference isotope. Dose factors are obtained from DOE/EH-0071 (Reference 10); the W solubility class is used since the plutonium is in nitrate solution. This calculation gives the curie equivalency of the isotope. These are then multiplied by the ratio of the activities of the isotope being evaluated and the reference isotope to determine the mass of the reference isotope that is equivalent to the mass of the isotope being evaluated. Since the activity of an isotope is (0.693/t1/2) where t1/2 is the half-life of the isotope, the inverse ratio of the half-lives can be used in lieu of the ratio of the activities. Since each isotope is converted to a mass equivalency of the reference isotope, the individual results can be summed. Mathematically, this is expressed in Equation 4 below.
There are three tanks in each line (six total) that can potentially contain hydrazoic acid during processing. These are the two feed adjust tanks (NT-41 and NT-42), the precipitator tanks (NP-1/2 and NP-3/4), and the filtrate tanks (NT-51 and NT-52). Processing solution is moved from one tank to the other during processing, so it is highly unlikely that processing solution would be in more than one tank at a time. As a bounding assumption, it is assumed that all six tanks have a bounding batch (1,500 grams) of plutonium solution in them simultaneously, that all six tanks simultaneously have explosive mixtures of hydrazoic acid present, and that all explode. If this happened, the source term would be 9,000 grams of plutonium. The isotopic composition of the plutonium solution, and of the LAP material, is given in Reference 7. Table 2 below shows the gram equivalency of the LAP source, and Table 3 shows the gram equivalency of the plutonium solution source. Note that it is conservatively assumed the plutonium solution source term contains Am-241, even though this material is separated from the plutonium by ion exchange.
Table 2. Pu-238 gram equivalent of hydrogen explosion source for LAP
|
ISOTOPE |
WT |
HALF-LIFE, |
DOSE |
PU-238 |
|
FRACTION |
YEARS |
FACTOR, |
EQUIVALENT, |
|
|
REM/m CI |
GRAMS |
|||
|
Pu-238 |
0.85 |
8.77E+01 |
4.60E+02 |
2.54E+03 |
|
Pu-240 |
0.15 |
6537 |
5.10E+02 |
6.67E+00 |
|
TOTAL |
2.55E+03 |
Table 3. Pu-238 gram equivalent of hydrazoic
acid explosion for Phase II processing
plutonium solutions (9,000 grams of plutonium in solution)
|
ISOTOPE |
WT |
HALF-LIFE, |
DOSE |
PU-238 |
|
FRACTION |
YEARS |
FACTOR, |
EQUIVALENT, |
|
|
REM/MMCI |
GRAMS |
|||
|
Pu-236 |
1.00E-06 |
2.85E+00 |
1.60E+02 |
9.64E-02 |
|
Pu-238 |
0.01 |
8.77E+01 |
4.60E+02 |
9.00E+01 |
|
Pu-239 |
0.65 |
24110 |
5.10E+02 |
2.36E+01 |
|
Pu-240 |
0.28 |
6537 |
5.10E+02 |
3.75E+01 |
|
Pu-241 |
0.03 |
14.4 |
1.00E+01 |
3.58E+01 |
|
U-232 |
1.00E-09 |
7.20E+01 |
1.30E+01 |
3.10E-07 |
|
Am-241 |
0.03 |
432.2 |
5.20E+02 |
6.20E+01 |
|
TOTAL |
2.49E+02 |
The LAP hydrogen explosion source term is (2,550/249) ~ 10 times higher than the hydrazoic acid source term. For the dose from a hydrazoic acid explosion to have the same consequences as the LAP hydrogen explosion, the energy released per mole of reactants would have to be ~ 10 times that of the hydrogen explosion.
The heats of formation of the potential reactants and products for hydrazoic acid decomposition, and hydrazoic acid combustion reactions, are shown in Table 4. The heats of formation of elemental molecules (O2, H2, N2) is zero by convention. A negative heat of formation means energy was necessary to form the molecule. The potential reactions and the amount of energy liberated are shown in Table 5. Some reactions in Table 5 may not occur, but are listed for completeness.
Table 4. Heat of Formation of Key Compounds
|
COMPOUND |
HN3 |
H20 |
N20 |
NO |
NO2 |
N2O4 |
|
HEAT OF FORMATION, KCAL/MOLE |
70.3 |
-57.79 |
19.55 |
21.6 |
7.96 |
2.23 |
Table 5. Energy Generated by Hydrazoic Acid Reactions
|
REACTION |
REACTION ENERGY, KCAL/MOLE HN3 |
|
HN3 -> 1/2H2 + 3/2N2 |
-70.3 |
|
HN3 + 1/2O2 -> H2O + 3/2N2 |
-128.09 |
|
HN3 + O2 -> 1/2H20 + 3/2 N2O |
-69.87 |
|
HN3 + 7/2O2 -> 1/2H20 + 3NO |
+0.855 |
|
HN3 + 13/2O2 -> 1/2H20 + 3NO2 |
-75.215 |
|
HN3 + 13/2O2 -> 1/2H2O + 3/2N2O4 |
-95.75 |
The most energetic reaction is the second one listed. This may occur as a two-step reaction, with the hydrogen liberated by decomposition then reacting with oxygen. The energy in a hydrogen explosion, per gram-mole of hydrogen, is –59.97 kcal. The energy released per mole of hydrazoic acid can be (-128.09/-57.79) ~ 2.2 times greater than from a hydrogen explosion. This is less than a factor of 10 times the energy of the hydrogen explosion, so the LAP hydrogen explosion consequences bound the Phase II hydrazoic acid explosion
This analysis shows the consequences for a hydrazoic acid explosion in vapor are bounded by the results from a hydrogen explosion. If a quantitative analysis of the consequences of a hydrazoic acid explosion is performed, the quantitative results should be used for evaluating the consequences of a hydrazoic acid explosion, rather than the hydrogen explosion results.
There is no analogous calculation for explosive liquids in the LAP analyses, so the consequences of a hydrazoic acid explosion in liquid cannot be compared to a similar LAP analysis. Since the frequency of a hydrazoic acid explosion in liquid is BEU, a separate calculation will not be performed.
5. Assumptions
The following assumptions were made to allow use of the LAP hydrogen explosion consequences as bounding for the hydrazoic acid explosion.
The following assumption was made to provide a bounding case for the inventory subject to a hydrazoic acid explosion.
The following controls on hydrazine addition were assumed to estimate the frequency of a hydrazoic acid explosion in the vapor phase.
The following control on hydrazine addition was assumed to estimate the frequency of a hydrazoic acid explosion in the liquid phase.
6. Discussion
The basis for assuming that controlling hydrazine concentration to 0.15M maximum was established in the 1950’s. A review of literature since the 1960’s demonstrated that the control values chosen then were very conservative. Applying the historical controls makes an hydrazoic acid explosion in both the liquid and vapor phase of process vessels BEU. But, if an explosion did occur, the consequences of the explosion would be less than for the hydrogen explosion assumed in the current Authorization Basis documents.
7. Recommendations
It is recommended that all of the conditions listed in the Assumptions section be implemented in HB-Line Phase II AB documentation, and that the Functional Classification Report include the appropriate entries to implement the assumptions.
8. References