WSRC-TR-2000-00406
The Effect of Alkaline Earth Metal on the Cesium
Loading
of Ionsiv® IE-910 and IE-911
F. F. Fondeur, T. Hang, D. D. Walker, W. R. Wilmarth, and S.
D. Fink
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Summary
This study investigated the effect of variances in alkaline earth metal concentrations on cesium loading of IONSIV® IE-911. The study focused on Savannah River Site (SRS) "average" solution with varying amounts of calcium, barium and magnesium.
Keywords: Magnesium, Calcium, Barium, Cesium, distribution coefficient, ion exchange, Salt Waste Processing Facility, Crystalline Silicotitanate
Introduction
The Savannah River Site (SRS) continues to examine three processes for the removal of cesium from high-level waste. One option involves the use of crystalline silicotitanate (CST) as a non-elutable ion exchange medium. The process involves adding CST in its engineered form - IONSIVÒ IE-911 made by UOP, LLC. - to a column and passing the liquid waste through the column. Cesium exchanges with sodium ions residing inside the CST particles. The design disposes of the cesium-loaded CST by vitrification within the Defense Waste Processing Facility.
The liquid waste at SRS contains various amounts of alkaline earth metals. Alkaline earth metals may compete with cesium for the available sites on the sorbent, thereby detracting from performance. However, not all the alkaline earth metals exist in the waste. For example, there is not beryllium yield from fission or from any alpha, beta or gamma decay. Both beryllium and radium exist in very small concentrations in the Tank farm. Previous analyses show low concentrations of strontium in samples of liquid waste. Strontium enters as both fission yields and tramp contaminant in added process chemicals. Calcium enters as a contaminant primarily from well water and is also added in the de-fluorination of plutonium. Barium derives from cesium decay. The authors could not identify any published analytical measurements for magnesium, calcium and barium of the liquid waste. In fact, no solubility prediction of calcium, magnesium or barium in liquid waste appears to exist. The only relevant solubility determination of these metals occurred in a simplified solution matrix.1
The alkaline earth metals exit as mono-hydroxides in alkaline media. Therefore, these ions are singly charge and are surrounded by a hydration shell. When the alkaline earth ions shed their hydration spheres, they can move into IE-911 and compete with cesium for sites. Bostick et al. showed, in a previous study of IE-911 contacted with groundwater, calcium displaced cesium from IE-911.2 Based on this evidence, the Salt Processing Project requested a study of the impact of alkaline earth metals on cesium loading of IE-911.3,4 This study focus on the solubility of alkaline earth metals in simulated waste solution and their effects on cesium loading of IONSIVÒ IE-911 and on the precursor material IE-910.
Experimental
Solubility Test
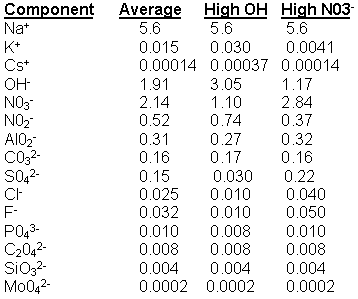
Before conducting the cesium removal tests, personnel determined the approximate solubility of magnesium, barium, and calcium in waste compositions of interest. Personnel prepared 1 liter of "average" salt solution5 with different amounts of MgCl2, BaCl2 or CaCl2 (corresponding to 0.01 to 0.001 molar concentration). See Table 1 for the composition of these solutions. During preparation, personnel maintained the concentration of sodium at 5.6 molar. Personnel prepared two solutions of 0.01 M CaCl2 in "Average" salt solution. One of the two solutions was mixed for 24 hours while the second solution was mixed for 48 hours. Samples were kept at laboratory temperature (22°C) Analytical results (ICP-AES) of both solutions indicate nearly identical calcium concentrations (5.4 and 4.6 x 10-5 M respectively). The lead researcher concluded 24 hours of mixing was sufficient to dissolve the soluble component of calcium in "Average" salt solution. Researchers mixed the solution for 24 hours filtered with a 0.2-micron nylon filter, and analyzed a portion of the solution for sodium, potassium, cesium and all the anions.
Table 1. Composition of SRS simulants.

IE-911 Pretreatment
Personnel placed 5 grams of IE-911 in a glass column suspending the sorbent on a #2 mesh filter. They passed distilled water in an up-flow motion through the sorbent bed at 4 cm/min until all the fines suspended above the bed disappeared. They then passed a 2 M sodium hydroxide solution in a down-flow motion at 4 cm/min until the equivalent of 40 bed volume flowed through the column. Personnel then passed distilled water again in a down-flow motion at 4 cm/min until the pH of the liquid exiting the column fell within the range of 9 to 10. At this point, personnel permitted the bed to drain, and placed in a dessicator until the sorbent reached a constant weight.
Kd Test
Personnel spiked portions of the simulants described above with 5 m Ci/L (137Cs).
They then added 20 mL of simulant to 0.1 grams of IE-911 in 60 mL bottles. The slurry shook for times as prescribed in the experimental plan at 150 rpm. Personnel filtered the slurry and scanned the filtrate for gamma radiation. The authors determined the amount of cesium loaded on IE-911 from the following expression.
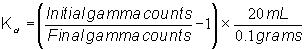
Results and Discussion
Solubility Measurements and Predictions
Personnel ran the OLIÒ software for average salt solution containing different amount of magnesium. Table 2 shows the results. The table also includes ICP-ES data for the simulant prepared with more than 0.01 M Mg. As seen from Table 2, the software predicts the Mg solubility in "Average" simulant relatively accurately. The solubility of Mg proves so small that it requires no further consideration. Furthermore, magnesium preferentially precipitates as magnesium hydroxide owing to large concentrations of hydroxide in the tank wastes.
Table 2. Measured and predicted solubility of Mg in SRS simulants.
Personnel ran the OLIÒ software
for and measured the calcium (Ca) solubility in "average" waste solution.
Table 3 shows these results. 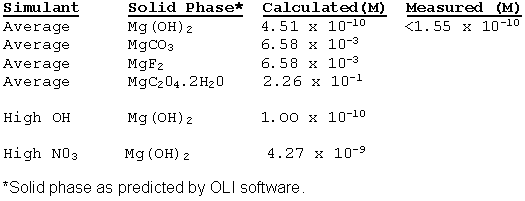
Table 3. Measured and predicted solubility of Ca in SRS simulants.
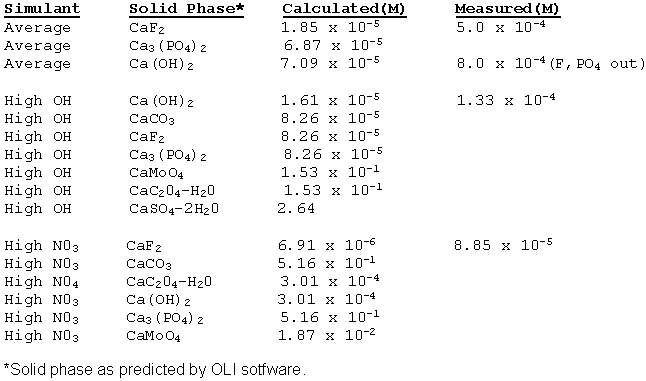
Looking at Table 3, one notes the software predictions did not agree with the measured value. The authors believe the OLIÒ software may not have sufficient information on calcium. Of the alkaline earth metals examined, calcium exhibited the highest solubility in "average" simulant (5 x 10-4 M). Table 3 also provides the measured calcium solubility in a simulant with no phosphate and fluoride. As predicted by OLIÒ software, although not as accurately, calcium solubility increased in an average simulant with no added fluoride and phosphate.
Personnel also measured barium (Ba) solubility and obtained predicted values from the software. Table 4 shows the results. Table 4 indicates barium exhibited lower solubility (one order of magnitude) than Calcium in "average" simulant. The OLIÒ software predicts barium solubility within one order of the measured value in SRS simulant.
Table 4. Measured and predicted solubility values of Ba in SRS simulants.
Cesium Distribution Coefficient Test
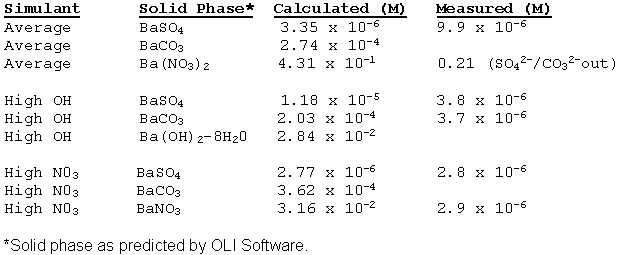
Researchers investigated barium effects on cesium loading. Personnel exposed six samples of IE-910 to simulant containing barium and another six samples to "average" simulant. The samples shook for 5 days. Figure 1 displays the results. The barium concentration dropped from 9.9 x 10-6 M to < 1 x 10-7 M. The authors believe barium absorbed onto IE-910. Looking at Figure 1, CST in "Average" salt solution had a mean distribution coefficient of 1735 ml/gcst with a standard error of 142 mL/gcst. CST in "Average with Barium" has a mean distribution coefficient of 1866 mL/gcst with a variance of 163.7 mL/gcst. The difference in mean is only 131 mL/gcst. This quantity is less than the variance of either test. Therefore, statistically the data provided no evidence of barium affecting cesium distribution coefficient.
Personnel shook a sample of IE-911 with a simulant containing calcium for 72 hours. They then injected the solution with cesium. Figure 2 provides the cesium-loading curve of this experiment. Cesium loads on IE-911 up to 1700 mg/L after 100 hours. At the same time the calcium level drops in solution during cesium loading. The calcium concentration reached a level below instrument detection and therefore, no steady state level was seen.
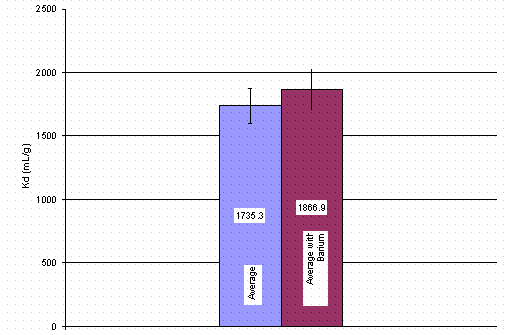
Figure 1. Barium Effects on Cesium loading of IE-910.
The decrease in soluble calcium represents an unexpected result. This decrease may result from simultaneous loading of both calcium and cesium. If both metals load onto the sorbent simultaneously, the ultimate cesium loading should prove lower than in the absence of calcium in solution. The final cesium Kd value equaled 1726 mg/L. As seen later in this report, this value does not differ appreciably from that determined during a test with calcium-free simulant (i.e., 1620 mg/L as shown in Figure 3). Therefore, the authors conclude calcium precipitation most likely accounts for the decrease in calcium concentration with time. The researcher designed the calcium experiment to conclude for 15 days. Cesium reached steady state after 11 days. Personnel immediately injected calcium (as calcium nitrate) to the samples. Looking at the four data points (24 hours apart in Figure 3), a small decrease in cesium Kd resulted. ). This reduction in cesium loading is not statistically significant. The final cesium Kd equaled 1566 mL/g.
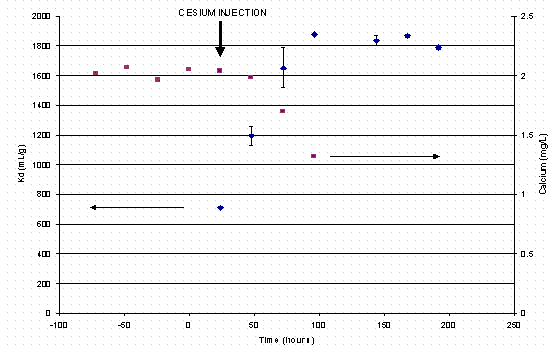
Figure 2. Cesium loading on IE-911 in Average simulant containing calcium. of IE-910.
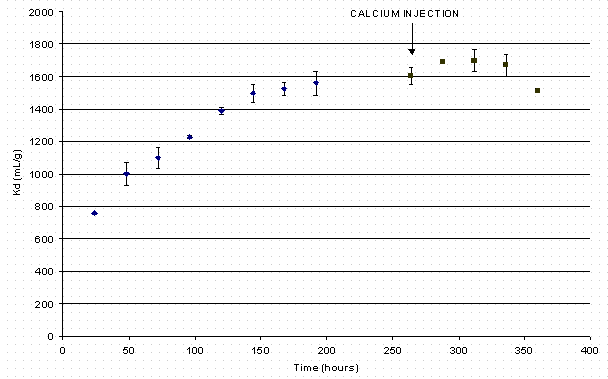
Figure 3. The effect of a calcium injection on the steady state cesium loading (IE-911). of IE-910.
When personnel simultaneously loaded both cesium and calcium on IE-911, the cesium-distribution coefficient curve (see Figure 4) proved similar to loading in the absence of alkaline earth metals (see Figure 2). This data indicates no detectable influence of calcium on the cesium distribution coefficient.
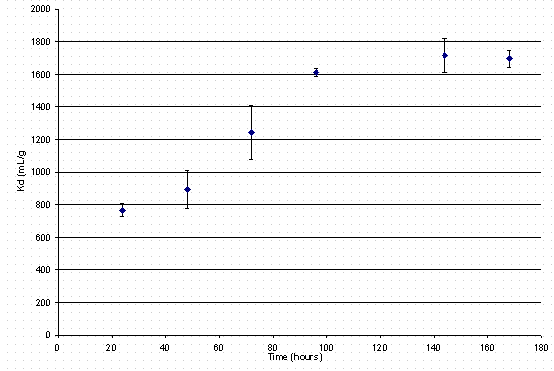
Figure 4. Cesium loading on IE-911 in the presence of calcium. of IE-910.
Personnel then performed experiments examining the influence of calcium on cesium loading of IE-910. This experiment investigated whether calcium influences the base CST material (i.e., IE-910) in the absence of any binder. Figure 5 shows the results for 12 samples, six contacted with simulant containing no added calcium and six with calcium. According to Figure 5, the presence of the calcium lowered the cesium Kd in IE-910 by 12%. This reduction is statistically significant as determined by the F-value of the six samples that included calcium in the simulant. In contrast, the ZAM model6 predicts no influence of calcium on the cesium loading at these conditions.
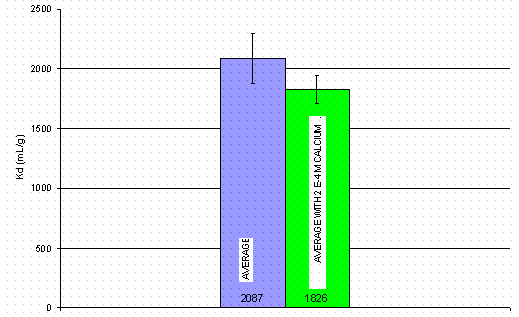
Figure 5. The effect of calcium on cesium
loading of IE-910. Half of the error
bars represent one standard error.
Personnel obtained the Langmuir constants, total capacity and equilibrium constant, from the lowered Kd value of the IE-910 in calcium-containing simulant. Table 5 provides the results of the calculation.
Table 5. The effect of calcium on cesium loading of IE-910.
Half of he error bars represent one standard error.

We calculated parameters "a" and "b" assuming linear (i.e., Langmuir) behavior over the entire cesium concentration range of interest. The expressions for the calculations follow.
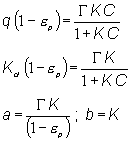
In this expression, e p is IE-910 porosity set at 0.24.6 The symbol "G " stands for the maximum cesium loading in IE-910 set at 0.6 mMol/g of CST.6 The symbol "K" stands for the equilibrium constant and is obtained from the formula given the Kd value.
Personnel then proceeded to predict the cesium loading curve from the VERSE program7 using the "a" and "b" values calculated in Table 4. Figure 6 displays the result. The presence of calcium at 5 x 10-4 M slightly lowered the amount of cesium loaded on the column. The difference proves smaller than any detectable variance given the analytical noise of the measuring technique. Since the impact of calcium proved minimal, the authors did not proceed with a column test.
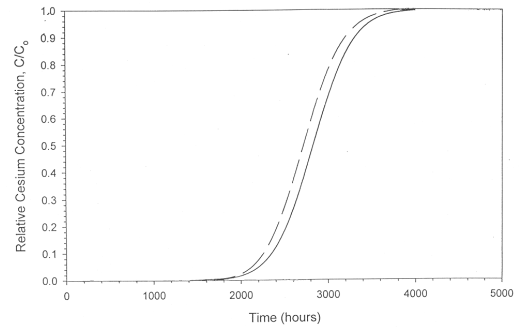
Figure 6. The cesium loading curve of IE-911
in the presence of calcium.
The dash curve shows loading in the presence of calcium.
The solid line shows loading with no calcium.
Conclusions
This work focused on the solubility of alkaline earth metals (i.e., magnesium, calcium, and barium) in SRS simulants and possible effects on cesium loading on crystalline silicotitanates (IE-910 and IE-911). Predictions (OLI® software) and experimental results indicated calcium has the highest solubility of the alkaline earth metals in simulated "average" SRS waste. Testing indicated barium did not affect cesium loading on granular CST (IE-911). Measurements indicated that the presence of calcium lowered batch Kd by 12% for powdered CST (IE-910), but not for the granular material (IE-911). Column calculations based on IE-910 indicate that the relatively small effect of calcium would not produce detectable changes in column performance for IE-911. Consequently, we did not perform column testing to confirm batch test results.
References