WSRC-MS-2000-00331
Characterization of Mixed Beta/Gamma Surface
Contamination
Using Passive Radiation Measurements
R. C. Hochel
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Abstract
The use of beta electret ionization chambers to characterize surface contamination has been suggested but, to date, not demonstrated and published. Work presented in this paper advances to practice a viable scheme of using passive beta EICs, in combination with a smattering of simple sodium iodide detector measurements, to completely characterize b -g surface contamination in reactors or other facilities where the contaminant is expected to have an aged fission product distribution possibly commingled with Co-60. If needed, passive EICs designed specifically for detecting tritium and a -activity have been similarly demonstrated, and may be added as a check for these contaminants.
These measurement devices far surpass the detection capabilities of standard radiological smears and probes, which have traditionally been used for characterization They are a superior survey tool to initially locate potential contamination and render a preliminary characterization for follow-up field measurements or occasional confirmatory sampling and more rigorous laboratory analysis. Used in conjunction with nuclear chemistry and process knowledge, they can dramatically simplify the challenge of characterizing many nuclear facilities.
Key Words: Radionuclides, Release Limits, Decommissioning, Waste Disposal
Introduction
There is a continuing and growing need for a simple and robust method which can be used to reliably characterize mixed beta/gamma radioactive surface contamination in facility and field environments. Undoubtedly, the biggest is in the emerging Deactivation and Decommissioning (D&D) business of facilities related to the nuclear power industry or nuclear material programs of the United States Department of Energy (DOE). These facilities are often large and contain many kinds of radioactive contaminated equipment and waste. But they also contain even larger amounts of uncontaminated materials that can be salvaged for reuse, or otherwise disposed of inexpensively as landfill waste or rubble. For such large D&D projects, many millions of dollars can be saved if a relatively simple in-situ method of characterizing any likely potential radioactive species can be devised.
This same need applies equally to the many such facilities which are still operating. The safety of personnel who enter or work in these facilities is largely the responsibility of the radiological control (or health physics) organization. Field instruments here are generally adequate for proper protection of personnel, but they cannot characterize found contamination beyond gross level and type. True characterization of mixed radioactivity often still remains a job of obtaining a proper sample for more detailed laboratory analysis. This is much more difficult and expensive if the unconditional release of materials is under consideration. The generator of material must provide for its defensible characterization and then request appropriate surveys and documentation by radiological control, which has primary responsibility and authority for regulated release options.
Further complicating the process is the lack of clear release criteria for most materials. Very few criteria dealing with material contamination in volume or bulk have yet been codified into federal regulations. Only surface contamination criteria exist (nominally 5000 dpm/100 cm2 beta/gamma and 100 dpm/100 cm2 alpha). A consensus is forming, both nationally and internationally, for comprehensive criteria for both types of contamination that are based on dose risk. But until this happens, there are defensible characterization options under current rules which can be pursued. Several of the available options here at the Savannah River Site (SRS) and other DOE facilities have been already been discussed by this author [1]. Many of the same options are proposed or in place at a D&D project at former small European nuclear power reactor and thoroughly described by workers Klein and Moers [2].
A nearly universal radiological characterization process employed in D&D and waste disposal has been used for years with little change. Identification of contaminated areas is made by systematic surveys by radiological control inspectors, often aided by process knowledge. Surface contamination is usually sampled by smears, while a true physical sample is sought to test for bulk contamination. Smears and physical samples are then sent to a laboratory for radiochemical analyses which are then used to estimate distributions of activities and isotopes for the identified contaminated areas and materials. Frequently, the distributions will include one or more gamma-emitters and these are then often used for further in-situ measurements to estimate facility inventory and to identify the need for or the progress of decontamination efforts.
This characterization process is widely accepted and mostly successful; however, it is not without some troublesome shortcomings. Smear samples, the established method of determining removable surface contamination, are unreliable, often missing one or more of the radionuclide present on the surface or especially in the bulk. Tritium, a common contaminant is many DOE facilities, is particularly difficult to detect by smears or probe surveys of concrete [3]. As a result, many more bulk samples are taken than might otherwise be necessary. This is costly, slow, labor and dose intensive, and often spreads contamination and generates secondary liquid waste. And in spite of the effort, the potential of missing some contamination, which further impacts D&D operations and disposal costs, is still possible because of inadequate or incomplete radiological surveys and sampling.
Better Technologies and Methods
An avoidance of such surprises must rely on more thorough in-situ measurements, which are more sensitive yet less demanding than some of the current practices. This usually involves bringing more sophisticated detectors and sensors to bear. Gamma-ray detectors, which are more sensitive and energy specific than those used for standard radiological probes, are an obvious choice. Inexpensive and portable sodium iodide detectors are popular. High purity germanium detectors offer much better isotope specificity, but are more cumbersome and expensive for field use. Their use is more essential when uranium and transuranium (TRU) contamination is important or when the activity in closed vessels must be estimated. Computer algorithms, which can model the response of any germanium detector to specific radionuclides contained in vessels of known dimensions and at various source-to-detector distances, are now offered for in-situ characterization [4]. These concepts have also been extended to field neutron measurements [5].
Simple In-Situ Detection Devices for Surface Contamination
Better in-situ characterization methods are not always more costly or difficult to use. Several types of small, inexpensive devices function in a passive mode where the device is placed on a surface, left for a few hours or days, and then collected for readings that quantifies the activity at the measured location. Three that have been used in D&D or environmental applications are the 1) alpha track detector (ATD), 2) thermoluminescent dosimeter (TLD), and 3) electret ion chamber (EIC).
ATDs are made of plastic that is sensitive to impinging alpha particles. The particles leave tracks in the plastic that can be chemically etched and counted under a microscope. They have the advantage of also showing the spatial location of the alpha contamination. They have little or no sensitivity to beta and gamma radiation. ATDs represent the near ultimate in alpha sensitivity, but do require laboratory processing [6].
TLDs are among the most common passive measurement devices. Traditionally, they are widely and almost universally used for personnel dosimetry. Radiation striking the dosimeter, usually a lithium fluoride crystal, produces exited luminescent electronic states that de-excite upon heating to a few hundred degrees. They are primarily sensitive to beta and gamma radiation, but have been adapted to neutrons as well. They are small and rugged and find use in point dose measurements, particularly in high radiation fields.
EICs consist of an electret which collects charge produced by radiation within an air-filled ion chamber. A variety of configurations are marketed by Rad Elec Inc.[7] which span a number of alpha, and beta (including tritium) monitoring applications. The electrets are statically charged devices that can be interchanged with different ion-chamber configurations. They respond to ionizing radiation, but otherwise retain their charge over long periods of time even in humid environments. Operationally, the electret voltage is read by a portable reader before it is mated to an ion chamber and deployed on a contaminated surface. After an appropriate exposure time, the electret is collected and the voltage is reread. The drop in voltage divided by the exposure time is a response that can be correlated to the surface activity by a calibration. The electrets, chambers, and voltage reader are relatively inexpensive and can be used over and over until the charge on an electret is spent and a new one must be purchased. By using an alpha EIC together with a beta EIC and a beta absorber, it is physically possible to discriminate between alpha, beta, and gamma activities. Field tests of the alpha and tritium beta EICs have been conducted in contaminated facilitates at SRS and found easy to use and much more sensitive than probing or smearing.
So far, there have been no technical reports of using EICs specifically for beta characterization other than tritium [3]. The challenge of doing so revolves about how to determine the radionuclide constituents in mixed beta-gamma contamination. The use of appropriate beta absorbers to determine beta energies is a solution if the spectrum is fairly simple, a few constituents at most. Slightly more complex spectra should be doable by coupling simple sodium iodide gamma measurements to those of EICs with and without beta absorbers.
Tomography and Imaging Methods
Over the last decade there has been a host of potential new characterization methods centering on tomography and imaging capabilities. Much of this has been aided by advances in the medical industry with the advent of CAT/MRI scans and three-dimensional x-ray imaging. The idea of imaging the specific location and activity levels within a facility or vessel is very attractive, but is also very complex and expensive compared with traditional methods. Applications are primarily in the TRU and high level waste areas where other alternatives are not available.
A very new and exciting exception is the application of so-called storage photostimulable phosphor imaging plates (SPP-IP) [8]. This technology too is a spin-off from the medical field. It is based on an image plate of a photostimulable phosphor (BaFBr:Eu2+) uniformly deposited onto a backing and covered by 10 m m of mylar. Any ionizing radiation incident on the IP produces excited electronic states in the Eu2+, some of which are phosphorescence and with the aid of light from a scanning laser, can be stimulated to de-excite. The phosphorescence is detected by a photo multiplier tube and is proportional to the type and activity of the incident radiation. By virtue of the high-Z elements, barium and europium, all types of ionizing radiation can be detected with remarkable sensitivity. IPs come in active areas up to 35 cm x 43 cm and have a dynamic range of more than 105. They are semi-flexible and could fit into large pipes or be placed on otherwise mostly flat regular surfaces. The IPs surface can be wiped clean of contamination and are also reusable after erasure by a UV source, which defrays their initial cost (~ $500/plate depending on size).
The sensitivities quoted in Ref. [8] are spectacular: a = 100 dpm/100 cm2, b = 1000 dpm/100 cm2, and g = 10 m R/h; all in a few hours of exposure time. Hot spots are easily identifiable from IPs and, with the use of a flux foil, even neutrons can be monitored down to 1 n× cm-2× s-1. Examples of some applications of this new technology are measurements of Cs-137 and natural radioactivity in granite and various foods and plants [9, 10, and 11].
As with the other passive devices above, measurements are a two step process: 1) exposure on the contaminated surface, and 2) reading of the plates and erasure for reuse, which is best done remote from the radiological area. Reading the IPs also requires some experience and expertise. Ref. [11] points out some potential problems in field applications that are not mentioned in the other cited references. The plates fade with time, they have to be protected from strong light sources after exposures, and for the best information, multiple readings of the IPs at different times are needed. Exciting as this technology appears to be, it is still too novel, expensive, and uncertain to recommend to the D&D community at this time without more study and testing.
The Case for In-Situ b-g Characterization
A compelling case can be made that the proper use of EICs in combination with any of a variety of portable sodium iodide gamma detectors can passively characterize surface and near-surface mixed radioactivity in many old nuclear facilities. This may seem an overly optimistic claim, but the methods and data presented in the remainder of this paper support this conclusion.
First of all, the situation is not nearly as complex and difficult as one might, at first thought, expect. Old nuclear reactor facilities and many others used in the processing of various nuclear materials exhibit a relatively simple distribution of radionuclides. The complex distribution of activities produced by the fission and the neutron activation processes decay to ones that are overwhelmingly dominated but by five or six activities: Co-60, Sr/Y-90, Cs-137/Ba-137m, and in some cases tritium. Co-60 arises from neutron activation of stainless steel, Inconel, and Zircaloy metals in or near the reactor vessel. Sr-90 and Cs-137 are long-lived fission products that occur in roughly the same relative amounts regardless of the type of nuclear fuel powering the reactor. Also since 64.1-hourY-90 is the short-lived daughter of 28.6-year Sr-90, the two must be in secular equilibrium and at equal activities. This is also true of the Cs-137/Ba-137m parent-daughter pair. Consequently, the five isotope distribution reduces to only three: Co-60, Sr-90, and Cs-137. Both Co-60 and Cs-137 via Ba-137m are easily measured with a sodium iodide detector, so their contributions to a beta EIC measurement can be subtracted leaving only the Sr/Y-90 beta response.
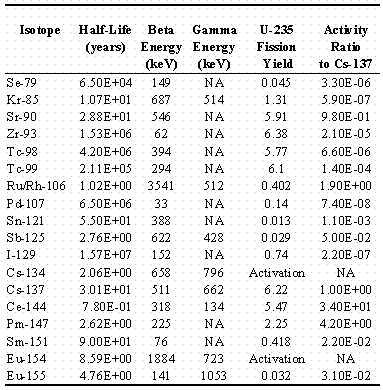
The possibility of some unknown beta emitters being present can be checked too. Sr-90 has a 546-kev beta end point energy, while that of Y-90 is 2284 keV. By calibration, the contribution from Y-90 may be unambiguously determined by using an absorber to completely block the Sr-90 or lesser energy betas. If the total activity measured by the bare EIC is not twice that of Y-90 measured by the EIC with the absorber, other beta activity must be present. Table 1 shows a listing of all the beta/gamma emitting isotopes with half-lives of 200 days or more that are possible as a result of U-235 fission.
Table 1. Beta/Gamma U-235 Fission Products Isotopes >
200 Days

A few minor isotopes possible from the neutron activation of stable fission products, half lives greater than 10,000 years, or tertiary fission tritium are excluded. Predominant beta endpoints and gamma rays are listed along with fission yields for each. Some values vary slightly depending on reference source, but none are significant for the purposes of the table. What is significant is the last column which shows the activity ratio of each isotope to Cs-137. They are easily calculated from the ratios of the respective fission yields and half lives in the table. Eight of the eighteen contribute far less than one percent of the Cs-137 and can be neglected for characterization purposes. Cs-134 (2.1 years) and Eu-154 (8.6 years) result from activation of natural isotopes produced in fission. Their ratios to Cs-137 are variable but are typically a few percent or so after 10 years of decay. A decade of decay also reduces Ru/Rh-106, Sb-125, and Ce-144 to negligible amounts. At that age, the only activities greater than one percent of the Cs-137 are Sr/Y-90, Cs-134, Pm-147, Sm-151, and Eu-154/155. The first three equally share ³ 75% of the total activity, with most of the rest as Pm-147. With the exception of weak beta emitters Sm-151and Eu-155, all are detectable with EICs. The contribution of four of the latter five, because of their relatively short half-lives compared to the first three, will continue to drop with time. While all could be ultimately be quantified by laborious laboratory analyses, the results would add no important new information not already obtained by EICs alone. Assigning all the variable excess activity, other than Sr/Y-90 and Cs-137, to a single worst case radionuclide would be conservatively safe and much simpler. Alternatively, these could be estimated from fission yields and age, with the gamma emitters Cs-134, Eu-154/155 verifiable by nondestructive gamma measurements if necessary.
Other Activation Products and the Actinides
A sizable number of neutron activation products and actinides are potentially possible in and around nuclear reactor facilities. Again, the characterization task seems daunting until some specifics are looked at more closely. Materials in close proximity to the reactor are primarily water, concrete, and various metals. Neutron activation of water produces some tritium from neutron capture by deuterium and a variety of activation products from dissolved materials and corrosion products, most of which are short-lived and trace in aged materials. Solid materials that absorb water appreciably are the major concern. Concrete is by far the main challenge. Activation products of concern in concrete are primarily K-40, Ca-41, and Fe-55. An increase in the natural K-40 content in concrete is easily detected by a gamma scan. Its presence would indicate a significant neutron exposure and the presence of Ca-41 and Fe-55 activities, which are otherwise not measurable by passive methods. Most concrete in reactor buildings has no appreciable neutron exposure, so tritium is the most likely radiological contaminant, and spent fuel storage basins are particularly susceptible. Electrets have been successfully used to detect tritium in concrete [3], so there are no in-situ measurement needs beyond the capabilities of EICs and sodium iodide.
Metals activated by neutrons must also be addressed. Aluminum, carbon steel, stainless steel, Inconel, and Zircaloy are the most common. The later three will all contain significant amounts of Co-60 if they have had any appreciable irradiation history. From this easily measurable activity, and the approximate decay time since irradiation, the activities of passively immeasurable isotopes Fe-55, Ni-59 and Ni-63 can be calculated. Activated carbon steel probably cannot be detected passively and some limited sampling and laboratory analysis might be necessary to characterize Fe-55 and possibly C-14. A small amount of Fe-55 is also possible in irradiated aluminum.
Similarly, nuclear physics and chemistry considerations rule out the presence of various actinides¾ in the absence of easily detectable fission products¾ on contaminated materials. A review of Ref. [12] or any comparable isotope generation and depletion code shows that, at most, actinide activity can account for only a few percent of the total activity in any reactor fuel or target. Furthermore, the solubility of most of the actinides in water compared to Cs-137 is quite small. Therefore, any surfaces exposed to contaminated water or air pathways will be dominated by Cs-137 contamination. But as a precaution, smears or alpha EIC measurements should be made on a few surfaces to confirm negligible actinide contamination.
Beta EIC Testing Needs
Descriptions of the characteristics of EICs and their principles of operation are reported in many papers extending back to 1980, but Refs. [13, 14, and 15] suffice to elucidate the main points of importance. Electrets are electrical analogs of a permanent magnet that carry a stable static electrical charge. They are mostly made from statically charged Teflon cut into disks of varying size and thickness. An electrically insulated housing holds the disk which is matched to a conducting enclosure of appropriate size to form an EIC. Construction is such that the electric field strength is constant and sufficient to produce ion saturation over a large voltage change as the initial charge is neutralized by ionizing radiation incident on the chamber. A portable reader may be used to measure the electret voltage at any time. By measuring the voltage change per unit time of a known constant-rate planar source, a calibration is established, which may then be used to characterize similar unknown sources. Such electrets have been shown to be remarkably stable even in humid environments as long as they remain capped and stored in areas of ambient background radiation.
It is useful to understand the physical principles behind the operation of different types of EICs. The electret is slowly discharged by the small ion current that results from radiation-induced ionization of air within the EIC and under the influence of the electretís electric field. EICs may or may not have a window to admit the radiation. The tritium EIC, for instance, has only an air window because the weak tritium betas would be stopped by all but the thinnest of solids. An alpha EIC has a very thin aluminized mylar window that will block weak betas but pass alphas and energetic betas. Beta EICs have a 7-mg/cm2 carbon coated Tyvek window. This shields alpha and most betas below about 70 keV. Alpha and beta radiation produce air ionization directly, but gamma rays only interact through the secondary electrons they produce in the chamber walls or active air volume. Accordingly, EIC are most efficient for alphas, followed by betas, with gammas only weakly detected.
Pretzch et. al. in Ref. 15 developed an expression for the response sensitivity, h , of cylindrical EICís defined as equal to DV/DtA, where DV is the change in electret voltage, Dt is the exposure time and A is the activity per unit volume or exposed surface area. An alternate expression for the response sensitivity based on theory was developed and physically verified as
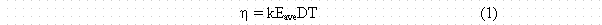
where k is a constant involving the electric field parameters and the effective surface charge density on the electret, Eave is the number of charge carriers of one sign created by the average energy beta particle, D is the distance from the electret surface to the base of the EIC, and T is the thickness of the Teflon electret disk. The expression holds as long as the voltage on the electret is sufficient to maintain saturated collection of the ions produced in the active volume. The response obviously increases with D, which is proportional to that volume. Less expected is that it varies directly with the thickness of the electret disk (due to space charge separation). The response also varies nonlinearly with the average beta energy of the particular energy spectrum being measured, due to changes in specific ionization with electron energy. Thus different beta emitters will have different responses. The small response to gamma rays also needs to be considered as it may contribute to EIC measurements.
To date there are no published reports on using beta EICs to characterize mixed beta activities on surfaces. Because the EIC response is expected to change from isotope to isotope, the specific response of each individual isotope must first be known or measured. The total beta EIC response will depend on which radionuclides are present in a given distribution and the relative amounts of each. Fortunately as discussed above, the distribution expected in most old reactor facilities is fairly simple in both the number of major constituents and their relative activities. So as will be shown, total activity and most of the individual radionuclides can be determined by passive measurements. This is simplified by the measured specific responses for a standard 50-cm2, 144-mL volume beta EIC and a standard 2"x2" sodium iodide (NaI) gamma detector given below.
Measurement Details and Results
Measurements of specific responses requires suitable individual or mixed radionuclide sources of known activities. Planar areal sources, each of 10 cm x 10 cm square, were purchased from Analytics, Inc. [16]. Four NIST-traceable sources were bought: Co-60 (2.26E+05 dpm), Sr-90 (2.18E+05 dpm), Cs-137 (2.37E+05 dpm), and Cs-137+Sr-90 (2.12E+05 + 2.11E+05 dpm). Each is covered with a thin (0.8 mg/cm2) Mylar film. The sources are each shipped with a thicker sealed outer plastic cover, which was removed and substituted with a resealable plastic bag. Before making measurements, the sources were removed form the bag to give the maximum beta emission. The activity levels were chosen to each be about 40 times the 5000 dpm/100 cm2 unconditional release guide. This allowed specific responses measurement to good accuracy with relatively short exposure times. The calibration accuracy for each of the sources was ± 5.0% at the 99% confidence level.
Electret Measurements
The electret response to each source was first measured in the normal mode, with the EIC placed directly on the unbagged source. The electret voltage was first measured with the reader device, screwed into a chamber, and then placed on the source at a recorded time. Exposure times were determined by stopping the exposure after a hour or two and checking the voltage drop and then continuing until the total drop was 50-100 volts. The uncertainty in electret voltage readings is ± 1-2 volts and the responses were therefore determined to within 5%, the same value as the source activity calibrations. Dividing the rate of voltage drop (volts/hour) by the exposed source activity gives the specific response for the measured radionuclide.
The sources were next measured through a 1.6-mm thick sheet of polycarbonate (190 mg/cm2). This was calculated as the absorber thickness necessary to stop betas with energies up to roughly 600 keV. Co-60 (b ep = 318 keV), Sr-90 (b ep = 546 keV) and Cs-137 (b ep = 512 keV) betas are all stopped by this absorber, and only the fraction of Y-90 (b ep = 2284 keV) betas over about 600 keV will pass through the absorber and be detected by the electret. Furthermore, any electret signal recorded in this configuration for the Co-60 and Cs-137 sources should represent just their respective gamma responses. The electret measurement data with and without the absorber are presented in Table 2.

Values in parentheses are estimated based on a ± 3 volts uncertainty is any measured response. The specific response for each nuclide is calculated based on the EIC window size of 50 cm2. Consequently only one half of each full areal source activity value noted above is used. The Co-60 activity was corrected for the slight decay from its calibration date (0.17 years). Also for Co-60 two gamma rays are emitted for each beta decay, so the specific response with the absorber is twice what it would be on a per gamma basis. Similarly, the activities which must be used for responses for Sr/Y-90 are twice the actual Sr-90 activity to account for the Y-90 daughter.
The specific responses for Cs-137 require some discussion. Its decay involves two beta branches with endpoints of 512 and 1173 keV and decay probabilities of 0.946 and 0.054 respectively. While the absorber can be expected to stop all of the former betas, some of the latter will obviously get through to the electret. And there is a further complication. The 662 keV gamma ray of the Ba-137m daughter is internally converted about 10% of the time, which gives rise to monoenergetic electrons of about 625 keV. Some of these probably get through the absorber as well. Finally, the conversion electrons add disproportionately to a higher electret response when there is no absorber because they are monoenergetic rather than variable as in the beta spectrum, which is characterized by a much lower average energy (nominally 1/3rd of the maximum end point energy). No attempt has been made to correct the Cs-137 responses for these effects.
The response data in Table 2 show some trends indicative of the physics involved. The electret response increases with beta energy up to perhaps 500 keV and then stays fairly constant. Comparing the specific responses with and without the absorber show that there is an apparent energy-independent response of about 3 percent to gamma rays (Co-60 and Cs-137), while about 20% of the total Sr/Y-90 betas pass through the absorber and are detected. Assuming all Sr-90 betas are stopped, about 40% of Y-90 betas pass through the 190 mg/cm2 absorber.
Gamma Detector Measurements
A 2"x2" NaI detector was chosen for the gamma ray measurements because of its small size and weight, high detection efficiency, inexpensive standard design, and simplicity of use. For field use, there are some disadvantages. Its relatively poor energy resolution makes separation of various gamma activities more difficult, and the effects of variable backgrounds and gain changes with temperature and count rate can be a problem. Still, the sensitivity advantage dominates as does the fact that results are directly applicable to any other 2"x2" NaI detector.
Measurement conditions in the laboratory were determined with the challenges of in-facility field conditions in mind. The K-40 in concrete, a ubiquitous building material, is used to advantage. Adjustment of the counting electronics so that the 1460 keV gamma ray of K-40 is at the high energy extreme of the spectrum allows an easy method of maintaining energy calibration under the many changing field conditions. Generally, only high levels of Co-60 will interfere with K-40 calibration, in which case the 1332 keV peak is an easily recognizable alternative. Three regions of interest (ROIs) were set ; 1) Cs-137: 557.9-749.8 keV, 2) Co-60: 1091.3-1267.1 keV, and 3) K-40: 1361.3-1540.2 keV.
Only gross ROI count rates were recorded. Attempts to use net photopeak rates were complicated by the relatively poor resolution of sodium iodide and the anticipated low signal to noise ratios that could exist under field conditions. Background corrections are instead determined from counts taken under similar ambient conditions where no surface contamination is present. The three ROI-background rates are then subtracted from those obtained from the contaminated surface. In similar manner, a correction to Cs-137 counts in ROI-1 from co-contamination of Co-60 can be made from the ratio of ROI-1 to ROI-2 measured with a known Co-60 source. Another significant correction to ROI-1 rates can occur if bremsstrahlung from Sr/Y-90 is not minimized. Use of a 3.2 mm thick polycarbonate absorber between the measurement surface and the detector reduces the bremsstrahlung contribution to ROI-1 substantially.
Each of the four planar calibration sources was counted through the bremsstrahlung absorber for 1000 seconds. Backgrounds were determined from longer multiple counts made on several different days. This was necessary to determine that the small Cs-137 and Co-60 background in the laboratory remained constant. The data are listed in Table 3. Each ROI rate is the net value after subtraction of background and any contribution from other radionuclides.
Table 3. 2"x2" NaI Gamma Measurement with 380 mg/cm2 Bremsstrahlung Absorber

Thus, the ROI-1 for the Cs-137+Sr/Y-90 source is the result of a gross count of 103.9 cps less the 10.5 cps background and the 0.7 cps bremsstrahlung from Sr/Y-90. The resulting specific response of 2.63E-02 compares favorably with that of 2.59E-02 for Cs-137 alone. The full 100 cm2 areal source activities are used here because the gamma detector, unlike the EIC, sees past its own footprint; however thanks to the near contact detector geometry, extending beyond the size of the100 cm2 calibration source to the much larger contaminated areas expected in the field, does not significantly alter the Table 3 values. The few negative numbers in Table 3 are a result of subtracting the Background ROI values from those obtained in the individual source counts. Their smallness is a measure of the constancy of the background in the laboratory measurement location.
Discussion of Results
Data in Tables 2 and 3 are additive and may be used to partially evaluate the validity of the proposed characterization measurement approach for contaminated surfaces. For instance, the Cs-137+Sr-90 source should give beta and gamma responses representing the sum of the two constituents. To see if this is so, assume that a hypothetical surface in an old reactor facility is measured with a beta EIC and gives a response of 39.5 vph during an initial survey. This is many times higher than background alone, so the surface must be contaminated. Next, a gamma scan of the same spot shows a Cs-137 peak with a net ROI-1 count rate of 92.7 cps after correction for K-40, the only other discernable peak in the spectrum. Is this sufficient information to characterize the surface radioactivity?
Simulated Composite Examples
The answer is yes if combined with a minimum of process knowledge about the facility. The most likely radionuclide distribution in an old reactor facility is one of fission products (Sr/Y-90 and Cs-137) and possibly activation products (Co-60). This can be mostly confirmed from a standard radiological survey of the area (smears and gross alpha and beta/gamma probes), but further characterization is needed to quantify the various activities. Using the NaI-measured Cs-137 activity and dividing its response of 2.59E-02 c/d from Table 3, the activity is computed as 3580 dps or 2.15E+05 dpm/100 cm2. From Table 2, one half of this activity (50 cm2 compared to the 100 cm2 total) should give an EIC response of 14.2 vph (1.07E+05x1.33E-04). Subtracting this from the actual electret response of 39.5 vph gives an excess of 25.3 vph. As no Co-60 was evident in the gamma scan, the residual activity is most likely due to Sr/Y-90. The Table 2 specific response for Sr/Y-90 is 1.22E-04 indicating the Sr/Y-90 activity on the surface is 25.3/1.22E-04 = 2.07E+05 dpm/50 cm2. So the activities per 100 cm2 by way of characterization are Cs-137 = 2.15E+05 dpm and Sr/Y-90 = 4.14E+05 dpm compared to the actual values of 2.12E+05 and 4.22E+05, respectively. The fact that the three activities are also nearly the same is further confirmation of an old fission product distribution, and consistent with process knowledge.
In instances of contamination solely from simple fission product distributions, electret measurements alone may suffice for characterization. This is usually the case for the large areas of reactor facilities that have been exposed to little neutron flux. Of the radionuclides produced in or near the reactor, those resulting from soluble fission vs. insoluble activation products usually pose the greatest potential for contamination to surrounding areas. So the dominant species after about a decade of decay will primarily be Sr/Y-90 and Cs-137. Again assuming the previous hypothetical example, beta electret measurements with and without the190 mg/cm2 absorber should be compared. If the surface contamination is expected to be relatively uniform, the two electret measurements may be made in parallel, otherwise successive measurements of the same spot should be made. Accordingly, the beta electret responses would be (from the bottom of Table 2) 39.5 vph without the absorber and 5.41 vph with the absorber. The 5.41 vph response implies a Sr/Y-90 activity of 2.27E+05 dpm (5.41/2.38E-05). The full electret response for this activity should be 27.7 vph (2.27E+05x1.22E-04). However, the true response is 39.5 vph. The difference of 11.8 vph must be attributable to other activity, which is most likely Cs-137. Using the Cs-137 specific response with no absorber from Table 2 would imply an activity of 8.87E+04 dpm. Although the sum of the three activities closely match the total source activity under the exposed electret (3.16E+05 vs. 3.17E+05), the distribution is skewed with Cs-137 about 20% too low (8.87E+04 vs. 2.27E+05/2). This is a result of not accounting for the small Cs-137 contribution to the with-absorber measurement. Two successive iterations of subtracting the calculated Cs-137 contribution from the with-absorber readings, and recalculating the Sr/Y-90 and Cs-137 values brings agreement between the three activities to within a percent. If iteration were to fail, an excess of other beta activity would be indicated signaling the need of further measurements.
Even easier than this method of characterizing contaminated surfaces is that of determining if a surface is free of contamination and eligible for unconditional release. The criteria for such release are that the surface be less than 5000 dpm/100 cm2 in beta/gamma and less than 100 dpm/100 cm2 for alpha. Taking the most conservative specific response from Table 2 of 8.52E-05 vph/dpm, 5000 dpm of virtually any beta/gamma surface contamination would give an electret response of about 0.2 vph. A change of less than five volts over a 24-hour exposure would likely indicate a surface does not exceed the release criteria, and longer exposures of several days could verify a reportable "less than value" to within about 15%. At such a point, authorized radiological personnel could perform their required surveys (including verification of no alpha or removable contamination) for documentation and formal release.
Composites of the Co-60 with only Cs-137 and with Cs-137+Sr/Y-90 were also simulated by summing equal length counts or exposures of the appropriate two sources. Assuming these composites again represented those from unknown contaminants, the beta and gamma data from Table 2 and 3 were used analogous to the Cs-137+Sr/Y-90 deconvolution above. Results, determined by gamma measurements, beta measurements or combinations of the two, again were typically within a few percent of the actual values. Thus to the extent these composites represent real field distributions, this characterization method is laboratory tested and applicable to the mixed radionuclides likely to be encountered in nuclear D&D operations. Considering real and hypothetical processes that can separate fission and activation products from the actinides or other nuclear materials, the complete scheme¾ electrets, gamma measurements, confirmatory smears, and a few samples¾ is nearly immune to characterization mistakes. Electrets detect the total surface activity and major beta emitters, gamma scans allow deconvolution of the mix beta distributions, smears verify the absence of alpha or excessive transferable contamination, and a few samples verify process knowledge and the passively-measured distributions. Short of drastically commingling widely different distributions or introducing significant amounts of unusual and unknown radionuclides, the scheme is self-checking against false assumptions and incomplete process knowledge.
Electret Response Considerations
Electret response is determined by the number of ion pair produced within the air space volume of the EIC. This is related to the specific energy loss of the beta particle in air, which is neither constant nor linear with beta particle energy. Instead it varies inversely and rapidly with energy up to about 50 keV, less rapidly between 50-500 keV, and then remains essentially constant from 500 keV to 10 MeV [17]. Because beta particle energies vary from zero to a maximum characteristic of the radionuclide, predicting electret response can be only approximate. Over a limited range of average beta energies of perhaps 100-1000 keV (Co-60, Cs-137, Sr-90, and Y-90), Table 2 shows EIC response is relatively constant. Fortunately, most beta emitters of interest fall in this range (see Table 1). Also, the interaction of most gamma rays within the EIC will produce electrons (Compton and photo electrons) within this range. However, the probability of a gamma ray interacting within the limited volume of the EIC is only a fraction of that of betas. But by using EICs of different sizes and window materials, virtually any and all radioactive surface contamination can be detected and quantified (pure electron capture nuclei, which are rare in special nuclear materials, produce only X-rays that electrets can seldom detect).
EICs are also able to detect radioactivity in the bulk of a material to a limited degree depending on the nature of the material. For nonporous materials, alpha response is limited entirely to the surface. Energetic betas, however, may be detectable to a depth of several millimeters in light metals. Beta detection is possible to a depth equal to the range of the betas in a particular material. This is because betas that just escape into air at the surface are still able to produce ionization, and at very low electron energies the number of ion pairs produced in the EIC increases dramatically (a 10 keV electron produces about 17 times as many ion pairs in air as a 50 keV electron) [17]. Concrete is much more anomalous. It is very porous and has a huge specific surface area. Studies of tritium in concrete showed that these weak betas could be detected up to about ten times their computed range [3]. This effect will likely hold true for more energetic betas as well, and detecting Sr/Y-90 and other energetic beta emitters to depths approaching a couple of centimeters in concrete may be possible.
Finally, although electret measurements are simple and result in very little personnel exposure, sole reliance on them for characterization is unwise. Random spot checks of 5-10% of contaminated areas found by electret surveys should be gamma scanned and smeared to verify that distributions are consistent and free of detectable alpha and transferable contamination. Some suitable fraction of these should actually be sampled to determine if there is contamination in bulk, especially if concrete or other porous materials are involved. EICs will respond somewhat to high energy beta emitters in air from radon progeny, and nearby Y-90 surface radiation that emanates from outside the walls of the device. This should be tested against a shielded EIC which prevents detection of betas that could otherwise pass through the side walls of the chamber. Also, reality checks are very important. Results that indicate other than what is expected must be thoroughly investigated and a plausible explanation found. This should at minimum involve resampling and/or reanalysis, as both are often the source of discovered discrepancy.
Conclusions
The use of beta electret ionization chambers to characterize surface contamination has been suggested but, to date, not demonstrated and published. Work presented in this paper advances to practice a viable scheme of using passive beta EICs, in combination with a smattering of simple sodium iodide detector measurements, to completely characterize b -g surface contamination in reactors or other facilities where the contaminant is expected to have an aged fission product distribution possibly commingled with Co-60. If needed, passive EICs designed specifically for detecting tritium and a -activity have been similarly demonstrated, and may be added as a check for these contaminants.
These measurement devices far surpass the detection capabilities of standard radiological smears and probes, which have traditionally been used for characterization They are a superior survey tool to initially locate potential contamination and render a preliminary characterization for follow-up field measurements or occasional confirmatory sampling and more rigorous laboratory analysis. They are suited to a wide range of potential contamination levels from dose hazardous down to innocuous unconditional release materials. EICs can be placed in minutes and left unattended for hours or days to achieve the required detection limit, so worker exposure is minimal. EICs are inexpensive and may be reused with only occasional replacement of spent electrets. Placement of 50-100 of them would assure economical, statistical, and representative coverage in large facilities, largely avoiding inadequate characterization and some potential costly surprises that can plague D&D work.
These new tools can greatly simplify and speed traditional characterization processes. But the D&D professional must always exercise caution, curiosity, and cleverness in characterization. Results should not resemble a potpourri of radionuclides. In most cases, a distribution recognizable as one or two out of a half a dozen or so possibilities should emerge. No radionuclide should appear in a proposed distribution unless its presence is supported by process knowledge or there is irrefutable analytical evidence for its inclusion. Equally important is the use of scaling factors (vectors) for the inclusion of trace nuclides, which may be of special regulatory concern and logically present but too low to measure. Finally, a nuclear chemist or someone with first hand process knowledge of prior facility operations should be consulted before proceeding far into dispositioning strategies. This is cheap insurance compared to potential costs of a characterization mistake or oversight.
References