WSRC-TR-2000-00289
Corrosion Evaluation of Waukesha Metal 88 and
Stellite Alloy 12 and 712
J. I. Mickalonis and A. W. Bowser
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Introduction and Summary
The High Level Waste Division requested the Materials Technology Section (MTS) to evaluate the use of Waukesha Metal 88 (WM88) and Stellite alloys 12 (S12) and 712 (S712) as materials of construction for slurry pumps. As candidate materials, WM88 was chosen for the tilt pad column bearings and S12 and S712 were selected for the impeller bearings. The Stellite alloys are cobalt-based alloys typically used for their resistance to both corrosion and wear. WM88 is noted for resistance to galling and seizing. These materials, however, had not been evaluated for use in high level radioactive waste, which have a high pH.
A series of electrochemical corrosion tests were performed in support of this evaluation to determine the general corrosion rate and corrosion characteristics of these alloys. The tests were conducted at room temperature in simulated three waste tank environments. For WM88, the test solution was inhibited water, which is commonly used in the tank farm. For S12 and S712, the test solutions were a simulated Tank 8 waste solution and a 3 M sodium hydroxide solution. The general corrosion rates of all alloys in these solutions were less than 0.1 mils per year (mpy). The alloys displayed passive behavior in these solutions due to the protective nature of their oxides.
Experimental Procedure
The series of electrochemical tests used to evaluate the corrosion characteristics consisted of the following: open-circuit potential (OCP) monitoring, linear polarization resistance (LPR), and potentiodynamic polarization (PP). The tests were conducted in the order given. The LPR and PP tests were based on ASTM G59 and ASTM G5, respectively [1,2]. The OCP was monitored to determine when the test sample had reached a quasi steady-state. Once stable, the LPR was performed to determine a general corrosion rate. Results from the potentiodynamic polarization were used to evaluate the corrosion behavior of the alloys.
These tests were conducted using a computer-controlled Princeton Applied Research potentiostat, Model 273A. The test samples were exposed in standard five-port Pyrex electrochemical test cells. Graphite rods were used as the counter electrodes and a Ag/AgCl electrode was used as the potential reference. LPR and potentiodynamic polarization all required an applied potential.
Nominal compositions of the alloys, which were used to calculate the equivalent weights for corrosion rate, were taken as follows. For S12, the composition was 29% Cr, 1.8 % C, 9% W, and 60.2% Co. For S712, the composition was 29% Cr, 1.85% C, 2.75% Fe, 9% Mo, 2.75% Ni, 1.25% Si, and 53.3% Co. For WM88, the composition was 72% Ni, 12% Cr, 5% Fe, 3% Mo, 4% Bi, and 4% Sn. These materials also contain less than 1% of other constituents such as carbon, silicon, manganese, phosphorous and sulfur.
The test samples were cut by electrodischarge machining from stock materials. The WM88 was cut from round wrought material. For the Stellite alloys, cast plates were used. The samples were cut as cylinders with a 0.564-cm diameter. These cylinders were mounted in cold-mount epoxy. Prior to mounting, a copper wire was attached to the sample using a silver-base epoxy. The sample surfaces were ground with a series of silicon carbide papers ending with 800 grit. Samples were rinsed with distilled water and ethyl alcohol.
The test solutions were a simulated Tank 8 waste solution, a 3 M sodium hydroxide solution and inhibited water. Standard reagent grade chemicals and distilled water were used to prepare the solutions. The solution compositions and average pH values are shown in the table below.
Table 1. Molar Composition Of Test Solutions
|
Anion |
Tank 8 |
3M NaOH |
Inhibited Water |
|
NO3- |
0.5 |
0.0 |
0.0 |
|
NO2- |
3.0 |
0.0 |
0.011 |
|
OH- |
2.7 |
3.0 |
0.01 |
|
Al(OH)4- |
0.1 |
0.0 |
0.0 |
|
CO3- |
0.4 |
0.0 |
0.0 |
|
pH |
11.25 |
12 |
12 |
The tests were conducted following standard laboratory practices and written instructions.
Test Results
Stellite alloys 12 and 712 were both corrosion resistant to alkaline solutions, which simulated waste environments. The alloys displayed an active-passive behavior, probably through the development of an oxide film. WM88 was resistant to the inhibited water due to its passive behavior. For all the alloys, the oxide provided for the low measured corrosion rates of less than 0.1 mils per year (mpy).
During the OCP, the potentials of the Stellite alloys stabilized to values near –0.3 V in approximately 30 minutes. S712 appeared to stabilize faster than S12 in the Tank 8 simulant and the 3 M NaOH solution. In the inhibited water, WM88 stablized in approximately two hours to a more electropositive potential. The nominal OCP values are shown in Table 2 for the alloys (ND indicates no data taken).
Table 2. Average Potentials of Waukesha and Stellite Alloys
|
Alloy |
Tank 8 |
3 M NaOH |
Inhibited Water |
|
S12 |
-0.340 |
-0.312 |
ND |
|
S712 |
-0.320 |
-0.355 |
ND |
|
WM88 |
ND |
ND |
-0.195 |
The LPR values were used to calculate a corrosion rate based on the following equation [3]:
The parameters are: B is the Stern-Geary constant (0.022 V), D is the density (8.53 g/cm3), A is the area (1 cm2), E is the equivalent weight (25.6 for S12, 25.3 for S712, and 29.25 for WM88), and R is the LPR value. Table 3 shows the measured R values and the calculated corrosion rates. The corrosion rates are significantly less than 1 mpy, which show that these alloys have an excellent corrosion resistance to the simulated waste and inhibited water solutions.
Table 3. LPR Test Results for Stellite and Waukesha Alloys
|
Solution |
Alloy |
LPR |
Corrosion |
|
Tank 8 |
S12 |
1.3E5 |
0.08 |
|
S712 |
3.4E5 |
0.03 |
|
|
3M OH |
S12 |
2.7E5 |
0.04 |
|
S712 |
7E4 |
0.14 |
|
|
Inhibit Water |
WM88 |
3.9E7 |
<0.01 |
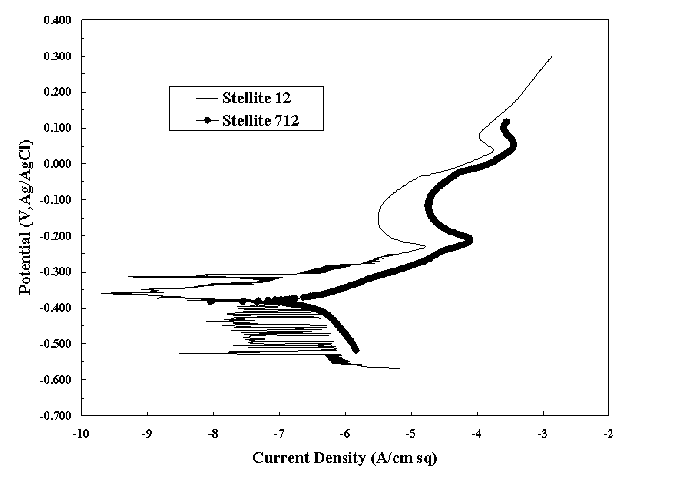
The PP tests for the Stellite alloys showed that they manifest active-passive behavior in the simulated waste. Figure 1 shows the polarization curves for S12 and S712 in 3M NaOH solutions at room temperature. The alloys displayed similar behavior, although S12 is at slightly lower current densities. The two alloys also displayed the active-passive characteristic in the Tank 8 simulant. The ranges of current density and potential were similar. Although not probable in service, the higher potential oxide that forms on these alloys appears to be less resistant than the surface oxide that forms under open-circuit conditions. This lower resistance is shown by the higher current densities.
The WM88 sample did not display active-passive behavior similar to the Stellite alloys. The WM88 alloy did not show a transition, but had a low passive current density of less than 1 m A/cm2. This behavior is indicative of the protective nature of the oxide in this inhibited water solution.
Conclusion
A corrosion evaluation was performed at the request of the High Level Waste Division for alternate materials of construction for Tank 8 slurry pumps. These materials were being considered for the bearings in the tilt pad column and the impellers. The candidate materials were Waukesha Metals 88 and Stellite alloys 712 and 12. Each of these alloys displayed passive behavior with corrosion rates less than 1 mpy for the various test solutions and should be resistant in the waste tank environment.

Figure 1. Polarization Curve For S12 and S712 in 3M NaOH Solution
References