WSRC-TR-2000-00103
Role of Edge Effect on Small Mammal Populations
in a Forest Fragment
F. D. Martin, and L. D. Wike
Westinghouse Savannah River Company
Aiken, SC 29808
L. S. Paddock
Savannah River Ecology Laboratory
University of Georgia
Aiken, SC 29802
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
In many cases, edge effect may determine the distribution and densities of small mammal populations. In 1995 and 1998, a mark and recapture study was conducted at the Savannah River Site (SRS), Aiken, SC, to evaluate the role of forest edge habitat. The area studied was an abandoned home site that had been recently isolated by a timber harvest. Harvest activities left a distinct edge of old field and planted pine contrasting with a relatively xeric, mixed hardwood stand. Trapping was conducted for 17 days in 1995 and 14 days in 1998. Three 30 m by 150 m grids were placed in the clear-cut, edge, and hardwood interior habitats. For both years the principal species captured were Peromyscus gossypinus, P. polionotus, and Neotoma floridana. The edge habitat accounted for approximately 55% of all captures and nearly four times as many recaptures as the interior and clear-cut habitats. In 1998, greater numbers of N. floridana were trapped than in 1995. Our results indicate that the use of edge habitat can be pronounced even within simple communities. Stewards of managed or restored habitats need to carefully consider the role of edge in these systems. In managed areas such as waste sites, movement of material within the food chain could be reduced by minimizing edge habitat around the points of contamination.
Keywords: edge effect, small mammals, habitat fragmentation
RUNNING HEAD: Edge effect in a forest fragment
During the past decade, the ecology of small mammals as considered from a landscape perspective has received increased attention (Kozakiewicz, 1993; Fahrig and Merriam, 1994). In recent years, landscape-level investigations have examined the effects of forest fragmentation and mammal dispersion in the clear-cut areas surrounding the forest fragments (Boone and Keller, 1993; Malcolm, 1994; Walters, 1991). These investigations have demonstrated that landscape structure has an effect on the dispersion and distribution of mammals in the area. In most cases, mammals were more abundant on the edge (e.g., the ecotonal area between the forest inclusion and the clear-cut areas) than in any other type of landscape feature investigated. Studies suggest higher density of game species on the edge may be due to greater complexity of vegetation and the availability of two or more habitat types. The species inhabiting the edge are provided with a greater amount of food and cover than they would obtain from any single type of habitat (Yoakum and Dasmann, 1969).
Further investigations have examined the availability of edge habitat on radioactive waste disposal areas (Boone and Keller, 1993). Results indicated that the majority of the small mammals inhabiting the waste disposal area preferred the edge habitat. By minimizing edge habitat in such areas, movement of material off the disposal sites through the food chain would be reduced and therefore lower the possible spread of contamination. The effects of landscape structure and vegetation pattern on movements of small mammal within and between landscape elements has important implications in the organization and regulation of populations (Szacki and Liro, 1991). Therefore, knowledge of habitat use by metapopulations can benefit resource management in clear-cut and fragmented areas and in risk management with waste sites.
Based on the results of previous investigations, our hypothesis was that the use of the edge habitat should be significantly greater than the use of either the hardwood interior or the clear-cut areas even for a very small hardwood stand.
Study Site
This research was conducted on the U.S. Department of Energy’s Savannah River Site located on the upper coastal plain of South Carolina. The study area consisted of approximately 40 ha of pine plantation with a hardwood inclusion of 0.72 ha. The area was previously planted with slash pine (Pinus elliottii) in 1948 and harvested in 1990. The clear-cut area was treated with herbicide in May of 1991 and then burned in October of 1991. It was replanted with longleaf pine (P. palustris) in February of 1992. The nearby uncut areas of pine plantation have two roughly parallel lines of hardwood trees which appear to be the remnants of old fence rows and two areas of hardwood trees located along ephemeral stream courses. These areas of hardwood are separated from the hardwood inclusion by 200-400 m across old-field habitat.
To examine the effect of landscape structure on small mammals, the study area selected was the hardwood inclusion, a roughly rectangular shaped old home site surrounded by the clear-cut area. Trapping was done initially in 1995, and again in 1998. The study was conducted to evaluate edge effect in the experimental area, and to provide a basis for evaluating changes in edge use over time.
Methods
The live-trapping investigation was conducted 4 through 21 April 1995, and again three years later, 21 May through 5 June 1998. The study area was marked into three rectangular grids, each placed in one of three different habitat types present at the study area: edge, hardwood interior, and clear-cut. Each grid had 3 parallel lines of 15 stations with 2 Shermanâ live traps set at each station. The three lines were approximately 10 m apart and the distance between stations along a line was approximately 10 m. While the same grids were used both years, there were two transect lines of traps placed in 1995. These each had 30 stations at 10 m intervals as with the grid trapping lines. All traps were baited with commercial birdseed mixture combined with oatmeal and were checked and rebaited daily. All captured animals were identified, weighed, sexed, and individually marked.
Kruskal-Wallis nonparametric analyses of variance were run on the data from the three grids to determine if there were differences between the numbers of captures in the different habitats. Additionally, species associations were examined using the variance test for species association (Schluter, 1984), 2 by 2 contingency analysis, and two indices of association. This was done using a computer program taken from Ludwig and Reynolds (1988).
Results
1995
Placement of the grids and transects as well as the locations, by species, of captures for 1995 are shown in Figure 1. A total of 6630 trap nights yielded 69 individual animals for 82 captures (including recaptures): 43 captures for cotton mice, Peromyscus gossypinus, 37 for oldfield mouse, P. polionotus, and 1 capture each for eastern woodrat, Neotoma floridana and hispid cotton rat, Sigmodon hispidus (see Table 1). Fifty-five percent ( n = 45 ) of all captures occurred in the edge habitat while only 32% ( n = 26 ) occurred in the clear-cut area and 12% ( n = 10 ) occurred in the hardwood interior. By species, P. polionotus accounted for all clear-cut captures, two interior captures, and nine edge captures. P. gossypinus accounted for eight interior and 35 edge captures. The lone N. floridana captured was in edge habitat.
Kruskal-Wallis nonparametric analysis of variance showed that, in each case and based on captures, P. gossypinus and P. polionotus showed preferences for one or another habitat type (Table 2). Based on mean rank values, combined data indicates a substantially higher use of edge habitat than either other habitat type. P. polionotus appears to avoid the hardwood interior habitat while using the edge and clear-cut areas nearly equally. P. gossypinus appears to prefer edge habitat over the other two types.
The overall index of association for the 1995 samples is 0.069 ( W = 3.86 where W is a statistic measuring significant deviation from 1.0, d.f. = 56, p > 0.5 ). However, contingency analysis indicates significant differences in locations of capture for P. gossypinus and P. polionotus ( c2(corrected) = 44.88, d.f. = 1, p < 0.001 ). The Dice index of association is 0.069 and the Jaccard index of association value is 0.036.
For P. gossypinus, 26.7% of captures in the edge habitat were recaptures while the three captures in the interior were all only captures. For P. polionotus, 15.4% of captures in the clear-cut were recaptures while all eight captures in the edge habitat were only captures.
1998
Placement of sampling grids and locations, by species, of captures for 1998 are shown in Figure 2. A total of 3780 trap nights yielded 20 animals for 43 captures (including recaptures): 1 Ochrotomys nuttalli, 1 S. hispidus, 9 P. gossypinus, 14 P. polionotus, and 18 N. floridana. Approximately 56% ( n =24 ) of all captures occurred in the edge habitat while only 26% ( n = 11 ) occurred in the clear-cut and 14% ( n = 6 ) in the hardwood interior (Table 1). By species, P. polionotus accounted for 12 clear-cut and 2 edge captures. P. gossypinus accounted for 5 interior and 4 edge captures. N. floridana accounted for 17 edge and 1 interior captures. S. hispidus and golden mouse, O. nuttalli each accounted for 1 edge capture apiece.
Kruskal-Wallis nonparametric analysis of variance again showed significant differences between capture rates in the different habitats (Table 3). Based on mean rank values, edge habitat was used preferentially by all species combined. P. gossypinus showed an avoidance of the outside area. N. floridana appears to have a strong preference for the edge habitat.
The overall index of association for the 1998 samples is 0.049 ( W = 1.46, d.f. = 30, p > 0.99 ). Contingency analysis indicates significant differences in locations of capture for P. gossypinus and P. polionotus ( c2(corrected) = 4.11, d.f. = 1, p < 0.05 ). The Dice index of association is 0.000 and the Jaccard index of association value is 0.000. Contingency analysis further indicates significant differences in locations of capture for N. floridana and P. polionotus ( c2(corrected) = 10.70, d.f. = 1, p < 0.005 ). The Dice index of association is 0.000 and the Jaccard index of association value is 0.000. On the other hand, contingency analysis indicates no significant differences in locations of capture for P. gossypinus and N. floridana ( c2(corrected) = 1.31, d.f. = 1, p < 0.2 ). The Dice index of association is 0.105 and the Jaccard index of association value is 0.056.
For N. floridana, 76.5% of captures in the edge habitat were recaptures while there was only one capture in the interior (a recapture of a specimen originally captured in edge habitat). For P. gossypinus, 40.0% of captures in the interior and 50% of captures in the edge habitat were recaptures. For P. polionotus, 16.7% of captures in the clear-cut were recaptures while both of the captures in the edge habitat were only captures.
Combined Years
A Kruskal-Wallis analysis of variance of the combined 1995 and 1998 data showed significant differences in habitat use (Table 4). Using mean rank sum, combined data for all species for both years showed an apparent preference for edge habitat over the other habitat types.
When both years are combined, P. polionotus shows a slight preference for the clear-cut area and an avoidance of the interior area. P. gossypinus shows a preference for the edge, but will inhabit the interior. N. floridana shows a strong preference for the edge habitat.
For the combined years, N. floridana had 72.2% of all captures in the edge habitat as recaptures, P. gossypinus had 25% recaptures in the interior and 29.4% recaptures in the edge habitat, while P. polionotus has 16% recaptures in the clear-cut.
Population estimates for 1995 were higher than for 1998 for both Peromyscus species (Table 5) with population estimates for P. gossypinus being about 15 times greater in 1995 and P. polionotus population estimates being about 6 times greater in 1995. The total population estimate for 1995 (both Peromyscus species combined) was about 140 individuals compared to about 23 (both Peromyscus species plus Neotoma) for 1998. Because of the large numbers of recaptures in 1998 the total number of captures was not proportionally reduced (82 for 1995 compared to 43 for 1998).
Discussion
We captured only four species of murid rodents during the first year of this study and a total of five species for the whole study. There are a number of reasons for this low number of species. The southeastern United States has low diversity of murid rodents, having only 17 species compared to 26 for the state of Texas (Burt and Grossenheider, 1976), which is at comparable latitude and has less surface area. In addition, forest fragments, such as the hardwood stand in this study, tend to support fewer native species than do larger stands (Andrén, 1994). Species with higher body mass and which are adapted to a specific habitat appear to be more sensitive to habitat fragment size than are smaller or more generalist species (Bennett, 1990). Despite connections through pine plantations, the hardwood forest fragment in this study has been isolated from other hardwood forest areas for more than 40 years. Bolger et al. (1997) found that habitat fragments’ loss of species is negatively correlated with both fragment size and length of time since isolation. Gottfried (1979) also found that fragment size was negatively correlated with number of species supported, but also found that distance to nearest continuous forest tract was also negatively correlated with numbers of species supported. Interestingly, McCoy and Mushinsky (1999) found that small fragments may support disproportionately large numbers of rare species despite having fewer overall species per fragment. All of these factors may have roles in restricting the number of species, though we are unable to determine which is the most important for this study.
Our site, since the clearcutting, has had no available corridor to facilitate movement of P. gossypinus from the nearest forests. Merriam and Lanoue (1990) report that P. leucopus, an ecologically similar species, which were habituated to forests were reluctant to travel through non-forested areas and virtually confined their movements to corridors of hedge rows and other areas with bushes and trees.
Because of the relatively high percentage of recaptures in 1998 for the three most common species we believe that we were sampling resident populations. The areas with no recaptures may represent areas with very small or transient populations or where home ranges are so small that encountering a trap and thus recapture is less likely. We could not distinguish among the possibilities with these data.
Peromyscus polionotus does appear to be primarily an inhabitant of open and edge environments. The two individuals in 1995 captured in the interior of the hardwood inclusion were caught at stations where there was no bushy undergrowth. Trees were widely spaced at these stations and the ground cover was grass and forbs.
The high population estimates for the two Peromyscus species in 1995 is consistent with patterns seen elsewhere in North America in that population densities of Peromyscus species tend to increase initially in clear-cut areas where coniferous forest have been harvested (Kirkland, 1990).
There is discussion currently about whether the equilibrium theory of island biogeography (MacArthur and Wilson, 1963) applies to terrestrial habitat fragments. It appears that at a regional scale this theory may apply (Cutler, 1991; Patterson and Brown, 1991). At smaller scales, island biogeography theory may be less applicable. Middleton and Merriam (1983), based on examinations of isolated woodlots, conclude that fewer than 10% of taxa in southern Ontario have patterns of distribution consistent with the island biogeography theory and they speculate that this is because the species in that area have evolved efficient mechanisms for medium distance movements. Rosenblatt et al. (1999), working with the small mammal fauna of forest fragments in Illinois, likewise believe that for that region the island biogeography analogy does not apply well.
One confounding problem with habitat fragments is that the smaller a fragment is, the greater the edge to interior ratio is. This would mean that edge effect is stronger in smaller patches than in larger patches. It appears that in our study there is a clear edge effect. This effect was noted by Bowers et al. (1996) for Microtus pennsylvanicus. In their study the females that lived at the edges of patches had larger body size and more frequent reproduction than did females with territories in interiors of patches. They concluded that edge habitat is of higher quality and can support higher densities of rodents. They also suggested that individuals with territories having edges would have fewer neighbors to interact with than those having interior territories, thus the energy spent in aggression is lower.
On the other hand, Yahner (1986) found that edge habitat may sometimes be avoided by small mammals. In forest fragments newly isolated by clearcutting, the white-footed mouse, Peromyscus leucopus, occurred in the interior of fragments at much higher densities than they did at the edges. However, under some circumstances white-footed mice use edge habitat readily (Cummings and Vessey, 1994), perhaps where there is a less sharp edge than that seen immediately after a clearcut. Yahner (1988) notes that some species may use one side of the edge between two habitats and not the other. In our study, cotton mice and woodrats used the part of the edge more into the forest than they did the edge toward the clearcut.
Conclusions
Although we are hesitant to make any broad generalizations from the limited data at hand, it appears that the edge was preferentially used by the 3 most common species of small mammals. These species are adapted to somewhat different habitats. P. gossypinus is a common species and tends to be found in wooded areas, especially deciduous forests, swamps, and river floodplains. P. polionotus inhabits sandy, fallow fields and other open early-successional areas like roadsides and field borders (Webster et al, 1985). N. floridana is often considered a woodland species, and at SRS it inhabits areas similar to those where P. gossypinus is found. The relatively long life span and elaborate houses built by this species might indicate that they tend to inhabit more stable habitats.
The statistical inferences are less clear cut than those built upon natural history characteristics. For the Peromyscus species and for combined years, edge is distinctly selected over interior use. However in 1998 when N. floridana was the dominant species of the edge, this preference may have been extinguished as P. gossypinus appeared to use the interior habitat slightly more than it did the edge. This is possibly driven by the larger and perhaps more competitive N. floridana displacing P. gossypinus from the edge. P. polionotus avoids the interior of the study area and differences between edge and clear-cut usage are slight. This species may prefer the clear-cut area but use the edge. P. gossypinus selectively uses the edge and avoids the clear-cut area. The fact that there is no significant difference in use between the interior and clear-cut areas in 1995, seems to demonstrate that, in the absence of N. floridana, P. gossypinus avoids both habitats in favor of the edge habitat, even though the hardwood interior should be suitable habitat.
The results of this study indicate that even small areas and simple small mammal communities can demonstrate distinct affinities for edge habitat even if the member species are not strictly limited to that habitat type. This means that stewards of waste sites, regardless of the size, must consider effects of available edge habitat when evaluating potential movement of constituents of concern into and through the trophic levels of the local ecosystem. Likewise, designers and managers of restored areas need to consider the potential advantages of providing edge habitat, even in limited quantities, within the systems they control.
Acknowledgments
The authors would like to recognize the assistance of M. H. Paller, J. D. Peles, H. G. Hanlin, S. D.Holley, W. M. Fulmer, H. M. Westbury, A. M. White, and T. J. Bombard. This paper was prepared for the Department of Energy under contract number DE-AC09-96SR18500, and facilitated through Oak Ridge Institute for Science and Education.
Literature Cited
Table 1. Summary of captures for both years. The "Indiv."
column represents
the numbers of individuals captured and marked while the
"Capt." column indicates the number of captures,
including recaptures.
|
Interior |
Edge |
Clear-cut |
Totals |
||||||
|
Year |
Species |
Indiv. |
Capt. |
Indiv. |
Capt. |
Indiv. |
Capt. |
Indiv.1 |
Capt. |
|
1995 |
N. floridana |
0 |
0 |
1 |
1 |
0 |
0 |
1 |
1 |
|
1995 |
P. gossypinus |
7 |
8 |
26 |
35 |
0 |
0 |
31 |
43 |
|
1995 |
P. polionotus |
2 |
2 |
9 |
9 |
25 |
26 |
36 |
37 |
|
1995 |
S. hispidus |
0 |
0 |
1 |
1 |
0 |
0 |
1 |
1 |
|
Year Totals |
9 |
10 |
37 |
46 |
25 |
26 |
69 |
82 |
|
|
1998 |
N. floridana |
1 |
1 |
4 |
16 |
0 |
0 |
4 |
18 |
|
1998 |
O. nuttalli |
0 |
0 |
1 |
1 |
0 |
0 |
1 |
1 |
|
1998 |
P. gossypinus |
3 |
5 |
2 |
4 |
0 |
0 |
3 |
9 |
|
1998 |
P. polionotus |
0 |
0 |
2 |
2 |
9 |
11 |
11 |
14 |
|
1998 |
S. hispidus |
0 |
0 |
1 |
1 |
0 |
0 |
1 |
1 |
|
Year Totals |
4 |
6 |
10 |
24 |
9 |
11 |
20 |
43 |
|
1Total number of individuals does not equal sum of individuals across habitats because some individuals were recaptured in different habitat types.
Table 2. Kruskal-Wallis analysis of variance of habitat use data, 1995.
|
Group |
Count |
Rank Sum |
|
|
All Species |
|||
|
edge |
36 |
2267.5 |
|
|
inside |
36 |
1674.5 |
|
|
outside |
36 |
1944.0 |
|
|
K-W Test Statistic = |
6.766 |
||
|
Chi-square with 2 d.f.. |
p < 0.034 |
||
|
P. polionotus |
|||
|
edge |
18 |
451.0 |
|
|
inside |
18 |
362.0 |
|
|
outside |
18 |
671.0 |
|
|
K-W Test Statistic = |
15.462 |
||
|
Chi-square with 2 d.f.. |
p < 0.001 |
||
|
P. gossypinus |
|||
|
edge |
18 |
687.0 |
|
|
inside |
18 |
482.0 |
|
|
outside |
18 |
315.0 |
|
|
K-W Test Statistic = |
21.132 |
||
|
Chi-square with 2 d.f.. |
p < 0.001 |
||
Table 3. Kruskal-Wallis analysis of variance of habitat use data, 1998.
|
Group |
Count |
Rank Sum |
|
|
All Species |
|||
|
edge |
16 |
528.0 |
|
|
inside |
16 |
289.0 |
|
|
outside |
16 |
359.0 |
|
|
K-W Test Statistic = |
11.004 |
||
|
Chi-square with 2 d.f.. |
p < 0.004 |
||
|
P. polionotus |
|||
|
edge |
16 |
364.0 |
|
|
inside |
16 |
320.0 |
|
|
outside |
16 |
492.0 |
|
|
K-W Test Statistic = |
11.011 |
||
|
Chi-square with 2 d.f. |
p < 0.004 |
||
|
P. gossypinus |
|||
|
edge |
16 |
416.0 |
|
|
inside |
16 |
440.0 |
|
|
outside |
16 |
320.0 |
|
|
K-W Test Statistic = |
5.624 |
||
|
Chi-square with 2 d.f.. |
p < 0.06 |
||
|
N. floridana |
|||
|
edge |
16 |
563.0 |
|
|
inside |
16 |
317.0 |
|
|
outside |
16 |
296.0 |
|
|
K-W Test Statistic = |
24.435 |
||
|
Chi-square with 2 d.f.. |
p < 0.001 |
Table 4. Kruskal-Wallis analysis of variance of habitat use data, 1995 and 1998 combined.
|
Group |
Count |
Rank Sum |
|
|
All Species |
|||
|
edge |
84 |
12169.0 |
|
|
inside |
84 |
9432.0 |
|
|
outside |
84 |
10277.0 |
|
|
K-W Test Statistic = |
14.272 |
||
|
Chi-square with 2 d.f.. |
p < 0.001 |
||
|
P. polionotus |
|||
|
edge |
34 |
1606.0 |
|
|
inside |
34 |
1349.0 |
|
|
outside |
34 |
2297.0 |
|
|
K-W Test Statistic = |
25.748 |
||
|
Chi-square with 2 d.f.. |
p < 0.001 |
||
|
P. gossypinus |
|||
|
edge |
34 |
2162.0 |
|
|
inside |
34 |
1833.0 |
|
|
outside |
34 |
1258.0 |
|
|
K-W Test Statistic = |
22.515 |
||
|
Chi-square with 2 d.f.. |
p < 0.001 |
||
|
N. floridana |
|||
|
edge |
16 |
562.0 |
|
|
inside |
16 |
318.0 |
|
|
outside |
16 |
296.0 |
|
|
K-W Test Statistic = |
24.238 |
||
|
Chi-square with 2 d.f.. |
p < 0.001 |
||
Table 5. Population estimates for the three most common species.
Population estimates were made using the method
of Schumacher and Eschmeyer (1943)
(in Ricker, 1975).
|
Species |
Year |
Estimated Population Size |
Estimated Minimum Population |
Estimated Maximum Population |
|
P. gossypinus |
1995 |
48.0 |
18.6 |
77.4 |
|
1998 |
3.1 |
0.8 |
5.4 |
|
|
P. polionotus |
1995 |
91.5 |
51.0 |
131.9 |
|
1998 |
16.0 |
9.8 |
22.2 |
|
|
N. floridana |
1998 |
4.3 |
0.7 |
7.9 |

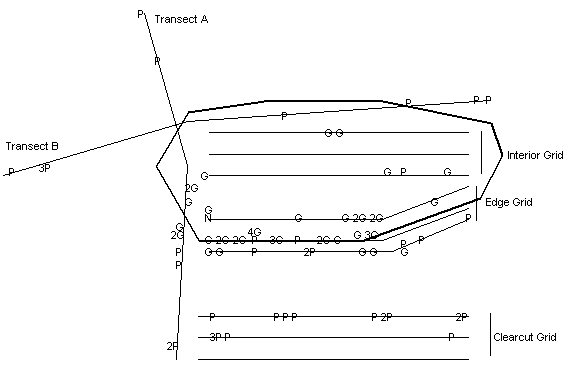
Figure 1. Diagram (not to scale) of the study area
showing sampling grids and transects for 1995.
Sampling grids are each 150 m in length while the transects are approximately
300 m in length. Letters indicate trap stations where individuals of each
species were captured. If more than one capture occurred at a station
the number preceding the letter indicates how many times.
G = P. gossypinus, N = N. floridana;
P = P. polionotus
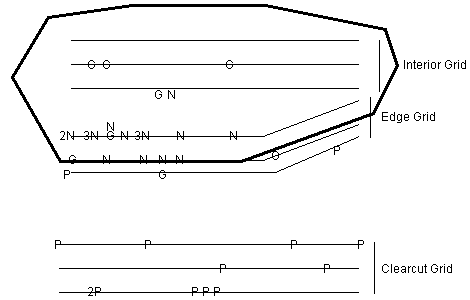
Figure 2. Diagram (not to scale) of the study area
showing sampling grids for 1998.
Sampling grids are each 150 by 30 m. Letters indicate trap
stations where individuals of each species were captured. If
more than one capture occurred at a station the number
preceding the letter indicates how many times.
G = P. gossypinus, N = N. floridana; P =
P. polionotus