Where g is 32.2 ft/sec2 and D is siphon inside diameter (ID) in feet.
WSRC-TR-2000-00066
Siphons for Geosiphon™ Treatment Systems
M. A. Phifer, R. L. Nichols, and F. C. Sappington
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department
of Energy and its contractors, in paper, from: U.S. Department of Energy, Office
of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062,
phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
1.0 Introduction
GeoSiphonTM systems (patent pending) induce contaminated groundwater flow through permeable treatment media by utilizing a siphon between two points of hydraulic head difference. A siphon is a closed conduit that conveys liquid from a point of higher hydraulic head to one of lower head after raising it to a higher intermediate elevation, at sub-atmospheric conditions (negative gauge pressures or vacuum), without external power input. All surface waters and groundwaters contain dissolved gasses, which degas within a siphon due to the vacuum and temperature within the siphon. Bubbles form, and if not properly managed will accumulate in the siphon, gradually reducing the flow rate until the system is ultimately shut down. Therefore appropriate management of gas within a siphon is the primary factor that must be considered to maintain continuous siphon flow. This report provides an overview of GeoSiphon technology and generic details concerning de-gassing in siphons and associated gas management methods.
2.0 Geosiphon™ Technology Overview
A GeoSiphon™ system is established by connecting a location of higher-pressure head to a location of lower-pressure head with a siphon to induce contaminated water flow through a permeable treatment media. The GeoSiphonTM is considered a passive remediation technology since pumps, compressors, etc. are not required to produce the necessary flow of contaminated water through the permeable treatment media. The first location with the higher-pressure head can be located within the contaminated portion of an aquifer or a surface water body. The second location with the lower-pressure head can be located within the same aquifer, another aquifer, the unsaturated (vadose) zone, the same surface water body, another surface water body, or the ground surface. The two points of hydraulic head difference do not have to be along the same natural groundwater or surface water flow path (i.e. they may not be naturally hydraulically connected). The two locations with a difference in hydraulic head are selected to provide the head difference necessary (substantial head difference) to drive the contaminated water through a permeable treatment media at the flow rate required.
The siphon, between the two locations, bypasses the natural porous media movement of groundwater within an aquifer by transferring the groundwater flow to the siphon. The siphon bypasses the natural resistance to groundwater flow inherent in the aquifer porous media and instead utilizes the natural energy for treatment. Bypassing the aquifer’s natural resistance to flow results in greater flow rates through the siphon than can be obtained naturally within the aquifer. This greater flow rate in the siphon in turn creates a cone of depression within the aquifer around the conduit inlet, increasing the gradient within the aquifer and subsequent flow within the aquifer toward the siphon inlet. This use of a siphon to bypass the natural groundwater flow serves both as the means to: 1) capture the groundwater contaminant plume, and 2) drive the flow of the groundwater through a treatment media at an accelerated rate over that which can be obtained by intersecting the contaminants with a trench of treatment media.
The inlet to the siphon, which is located at the first location with the higher pressure head, can be located within a trench, a sump, a horizontal well, a vertical well, a surface body of water, the permeable treatment media, or the permeable treatment media enclosure/housing. The siphon outlet, which is located at the second location with the lower pressure head, can be located within a trench, a sump, a horizontal well, a vertical well, a surface body of water, the permeable treatment media, the permeable treatment media enclosure/housing, or the ground surface.
Groundwater treatment using the GeoSiphon system is not limited to a specific waste stream or to a specific permeable treatment media. Appropriate permeable treatment media for use in GeoSiphon systems are selected to treat the contaminants associated with problem at hand. The permeable treatment media utilized in GeoSiphon systems can include materials such as but not limited to activated carbon, bimetallics, blast furnace slag, calcium peroxide, concrete, dolomite, fly ash, granular cast iron, ion exchange materials, iron, iron foam, lime, limestone, organic carbon, peat, phosphate rock, phosphates, pyrite, sodium carbonate, sulfur, and zeolites. The GeoSiphon permeable treatment media can be located at any point along the siphon; it can be located immediately prior to the siphon inlet, at any point within the siphon, or at the end of the siphon. The permeable treatment media can be applied as in situ or ex situ, it can be configured to be either permanent or rechargeable, and it can be contained within an enclosure/housing or in direct contact with the aquifer/soil materials.
Where enclosures/housings are utilized, they are designed to contain the permeable treatment media while allowing hydraulic communication to drive the flow in the siphon between the two locations with a difference in hydraulic head. Where the permeable treatment media is located in situ, hydraulic communication may be maintained through the use of a permeable enclosures/housings. The permeable enclosures/housings may utilize one or a combination of filter fabrics, GeoNets, GeoNets with filter fabric, geotextiles, gravel or rock, perforated casing, permeable membranes, rigid screens, etc.
Typically groundwater contaminants are highly stratified over the depth and width of the plume. This stratification is due to the natural stratification of soil layers (i.e. gravel, sand, silt, and clay), the location and geometry of the contaminant source, and the variations in subsurface hydraulic pressures. For GeoSiphon systems, which have the permeable treatment media located in situ at the inlet to the siphon, this stratification of contaminants, soil layers, and pressures requires that the thickness of the permeable treatment media be designed for the highest contaminant concentration layer entering the media. In this case design based upon the average contaminant concentration would result in the short-circuiting of contaminated water layer with greater than average concentrations through the permeable treatment media and thus insufficient treatment of this layers of contamination. The requirement to design the thickness of the permeable treatment media based upon the maximum aquifer concentration layers can be addressed in one of two ways:
Figures 1 and 2 show two typical GeoSiphon treatment system configurations. Figure 1 is a pre-siphon treatment cell configuration (i.e. treatment occurs prior to siphoning). Figure 2 is a post-siphon treatment cell configuration (i.e. siphoning occurs prior to treatment). Additionally treatment cells can be located at both end of the siphon.
[Phifer, et al., 1998; Phifer, et al., 1999a; Phifer, et al., 1999b; Phifer, et al., 1999c]
3.0 Siphon Technology
3.1 Siphon Technology Overview
A siphon is a closed conduit that conveys liquid (i.e. typically water) from a point of higher hydraulic head to one of lower head after raising it to a higher intermediate elevation, at sub-atmospheric conditions (negative gauge pressures), without external power input. A siphon has a maximum theoretical lift of 34-ft (equivalent to atmospheric pressure). However, it has a maximum practical lift of 25 ft due to the vapor pressure of water and friction head loss. [Gibson, 1961; Phifer, et al., 1998; Phifer, et al., 1999a; Phifer, et al., 1999c]
Siphons require priming (initial filling of line) to initiate flow. This can be accomplished by one of the following:
After priming, the siphon will convey liquid from the point of higher hydraulic head to the point of lower head so long as the head differential is maintained and the prime is not lost. [Gibson, 1961; Loitz, et al., 1990; Phifer, et al., 1998; Phifer, et al., 1999a; Phifer, et al., 1999c]
Accumulation of air can stop the siphon flow. However, this can be avoided by employing the following means:
[Gibson, 1961; Mathur, 1990; Phifer, et al., 1998; Phifer, et al., 1999a Phifer, et al., 1999c]
3.2 De-gassing in Siphons
All surface waters and groundwaters contain dissolved gasses, the concentrations of dissolved gasses depend upon the state of equilibrium with the atmosphere, the geochemical and biological processes which have occurred within the body of water, and the contaminants which have been released to the body of water. Surface water near the interface with the atmosphere is typically near equilibrium gas concentrations with the atmosphere. Deeper surface waters and groundwater, however are not typically near equilibrium gas concentrations with the atmosphere. Nitrogen and argon are typically considered conservative relative to fate and transport (i.e. only a few reactions occur which impact dissolved nitrogen and argon concentrations, therefore their dissolved concentrations are typically near those in equilibrium with atmosphere). Multiple reactions, both biological (aerobic microorganisms) and geochemical, occur which consume oxygen, therefore dissolved oxygen concentrations are typically depleted relative to that in equilibrium with air. Subsurface aerobic microbial respiration produces carbon dioxide in the subsurface. This results in subsurface carbon dioxide partial pressures ranging from 0.0003 to 0.1 atmospheres, which in turn results in dissolved carbon dioxide concentrations ranging from 0.6 to 192 mg/l. Subsurface anaerobic microbial and geochemical processes exist, which produce hydrogen and methane. Many other dissolved gasses may be present in surface water and groundwater, due to atmospheric and groundwater contamination. [Deutsch and Longmire, 1998; Phifer, et al., 1999c; Washburn, et al., 1999]
Table 1 presents dissolved gas concentrations associated with water in equilibrium with air, typical South Carolina coastal plain water table aquifers at the Savannah River Site (SRS), typical SRS groundwater which has contacted limestone, and typical SRS groundwater which has contacted granular cast iron (i.e. zero-valent iron). Table 1 also presents the make up of the bubbles produced from de-gassing of the above referenced groundwaters (i.e. the concentrations of the bubbles produced from de-gassing, which occurs within a siphon line transporting the referenced groundwaters).
Table 1. Dissolved Gas and De-gassed Bubble Concentrations
|
|
|
|
SC Coastal Plain Unconsolidated |
SC Coastal Plain Unconsolidated |
Limestone Contacted |
Limestone |
Granular Iron Contacted Groundwater Dissolved |
Granular Iron Contacted Groundwater |
|
N2 |
76.60 |
15.81 |
15.81 |
82.55 |
17.04 |
89.27 |
6.34 |
25.55 |
|
O2 |
20.55 |
10.25 |
5.13 |
11.07 |
1.74 |
3.77 |
1.01 |
1.68 |
|
CO2 |
0.03 |
0.59 |
96.3 |
5.39 |
68.08 |
3.82 |
1.51 |
0.07 |
|
Ar |
0.92 |
0.58 |
0.58 |
0.99 |
0.58 |
0.99 |
0.58 |
0.76 |
|
CH4 |
1.96E-04 |
5.0E-05 |
3.61E-04 |
1.53E-03 |
1.14E-02 |
4.83E-02 |
0.72 |
2.35 |
|
H2 |
4.90E-05 |
8.7E-07 |
4.62E-06 |
2.80E-04 |
1.86E-06 |
1.13E-04 |
1.45 |
67.98 |
|
C2H6 |
4.90E-05 to 9.32E-06 |
3.2E-09 to |
5.13E-06 |
8.53E-06 |
3.43E-04 |
5.72E-04 |
2.85E-03 |
3.66E-03 |
|
C2H4 |
6.87E-08 to 6.87E-05 |
1.3E-07 to |
6.02E-05 |
3.39E-05 |
3.50E-05 |
1.98E-05 |
6.70E-04 |
2.92E-04 |
|
H2O |
1.94 |
NA |
NA |
2.09 |
NA |
2.10 |
NA |
1.61 |
Notes to Table 1:
The dissolved gas in water concentration in equilibrium with air column is strictly based upon Henry’s Law and does not take into account potential carbonate series effects.
[Lide, 1998; Mirtov, 1964; Graedel, 1978; Roine, 1997; Streeter and Wylie, 1979; Deutsch and Longmire, 1998; Phifer, et al., 1999a; Phifer, et al., 1999c; Washburn, et al., 1999]
The dissolved gas contained within most groundwaters consists of the following major gasses (in typical descending order of concentration): carbon dioxide, nitrogen, oxygen, and argon. These dissolved gases subsequently result in de-gassed bubbles, which consist of the following (in typical descending order of concentration): nitrogen, oxygen, carbon dioxide, and argon. [Phifer, et al., 1999a; Phifer, et al., 1999c; Washburn, et al., 1999]
However groundwater, which has been treated by granular case iron, has a totally different dissolved gas composition. The following are the major dissolved gas reactions that occur due to groundwater contact with granular cast iron:
These changes in dissolved gas composition due to zero-valent iron result in a groundwater which contains the following major dissolved gases (in typical descending concentration): nitrogen, carbon dioxide, hydrogen, oxygen, methane, and argon. These dissolved gases subsequently result in de-gassed bubbles, which consist of the following (in typical descending concentration): hydrogen, nitrogen, methane, oxygen, argon, and carbon dioxide. [Phifer, et al., 1999a; Phifer, et al., 1999c]
Degassing within a siphon line will occur, and the continuous operation of a siphon line is essentially dependent upon gas bubble management. The volume of de-gassing within a siphon is dependent upon several variables including the specific dissolved gasses, the concentration of dissolved gasses, and the solubility of the gases under the conditions present. On a per mass basis hydrogen produces a larger de-gassed volume than nitrogen, and hydrogen more readily degasses than nitrogen. Therefore groundwater treated with zero-valent iron prior to transport within the siphon will more readily degas and produce a larger degas volume than typical groundwater. [Phifer, et al., 1999c] Gas solubility in water varies directly with the partial pressure of the gas in contact with the water and indirectly with temperature. [Manahan, 1991] Therefore, de-gassing within a siphon line increases with increasing vacuum and temperature.
Based upon these observations siphon degassing can be minimized by the following:
[Phifer, et al., 1999c; Washburn, et al., 1999]
3.3 Siphon Gas Transport
Management of gas within a siphon is of utmost importance to the maintenance of siphon flow. Siphon gas management requires control of gas bubble transport, accumulation and agglomeration, and elimination of gas bubble entrapment. Gas bubble transport, accumulation, agglomeration, and entrapment are controlled by bulk fluid flow velocity, gas buoyancy (i.e. gas type and bubble size), siphon diameter, siphon grades, and inside diameter discontinuities (i.e. fittings).
Gas bubble transport in the upward leg of the siphon is facilitated by higher bulk fluid flow velocities, a continuous upward siphon line grade (no localized high points), and the minimization or elimination of fittings, which produce discontinuities in the inside diameter of the siphon line. The continuous upward grade and elimination of such fittings promotes buoyancy transport in the same direction as bulk fluid flow and eliminates the accumulation, agglomeration, and entrapment of gas bubbles in the upward leg of the siphon line. The bulk fluid flow velocity in the upward leg is not as critical as it is in the downward leg of the siphon line, and the upward leg fluid flow velocity should be balanced against minimization of head loss to maximize overall flow rates.
The direction of gas bubble transport, if any, in the downward leg of the siphon is determined by whether the bulk fluid flow velocity or gas buoyancy dominate. Bulk fluid flow velocity tends to cause the gas bubbles to move downward in the downward leg of the siphon line toward the end of the line. Gas buoyancy tends to cause the gas bubbles to move upward in the downward leg of the siphon line toward the siphon crest. The desired direction of gas bubble transport within the siphon downward leg is dependent upon the method of gas management employed, that is whether air chamber gas management or minimum flushing velocity (MFV) gas management is employed. The preferred direction of gas bubble transport with air chamber usage is upward toward the siphon crest. The required direction of gas bubble transport with MFV usage is downward and out the siphon discharge.
Management of gas bubble transport in the downward leg of the siphon is more critical to maintenance of siphon operation and impacts the efficiency of operation more so than within the upward leg. Therefore in general it is recommended that the length of the upward leg be maximized and the length of the downward leg be minimized to the extent possible.
[Phifer, et al., 1999a; Phifer, et al., 1999c]
3.4 Air Chamber Gas Management
An air chamber is essentially a high point accumulation for gasses that de-gas from the water within the siphon (see Figure 3). Air chambers are located above the siphon at the line high point (crest) of the siphon and are connected to the siphon. The air chamber is initially filled with water during siphon priming. As the system operates and de-gassing occurs, the buoyancy of the gas and/or the bulk fluid flow transport the bubbles into the air chamber. The gas accumulates and displaces the water in the air chamber rather than with in the siphon itself, thus maintaining the siphon’s prime. The gas in the air chamber must be evacuated and replaced with water (recharged) on a periodic basis to maintain the siphon free of accumulated gas, which can reduce the flow rates and eventually break the siphon. The air chamber can be sized to minimize the frequency of manual recharging or an automated recharging system may be employed. [Gibson, 1961; Mathur, 1990; Phifer, et al., 1999a; Phifer, et al., 1999c]
Gas bubble transport in the upward leg of the siphon occurs toward the air chamber, which is located at the crest, due to buoyancy transport in the same direction as bulk fluid flow. This transport is facilitated by higher bulk fluid flow velocities, a continuous upward siphon grade, and the minimization or elimination of fittings, which produce discontinuities in the inside diameter of the siphon. The grade may be shallow, since buoyancy and bulk fluid flow transport is in the same direction, and localized high points should be eliminated, so that gas entrapment does not occur. However the bulk fluid flow velocity in the upward leg is not critical, since buoyancy and bulk fluid flow transport occur in the same direction. Therefore the velocity should be balanced against minimization of head loss to maximize overall flow rates. [Phifer, et al., 1999a; Phifer, et al., 1999c]
The preferred direction of gas bubble transport, with air chamber usage, within the siphon downward leg is upward toward the siphon crest and air chamber. Gas buoyancy tends to cause the gas bubbles to move upward in the siphon downward leg toward the siphon crest. Fluid flow velocity tends to cause the gas bubbles to move downward in the siphon downward leg toward the end of the line. For upward gas bubble transport in the downward leg, gas buoyancy and not the bulk fluid flow velocity must dominate transport, so that the gas bubbles migrate back up the siphon downward leg into the air chamber at the crest. This transport is facilitated by lower bulk fluid flow velocities, a continuous downward siphon grade, and the minimization or elimination of fittings, which produce discontinuities in the inside diameter of the siphon line. The grade should be as steep as possible, to facilitate buoyancy transport upward, and localized high points should be eliminated, so that gas entrapment does not occur. The diameter of the siphon downward leg can be sized to produce the required low, bulk fluid flow velocities (i.e. larger diameter downward legs will produce lower velocities). [Phifer, et al., 1999a; Phifer, et al., 1999c]
Based upon literature, the following empirical equation may closely represent the bulk fluid velocity, below which buoyancy transport in the siphon downward leg dominates (i.e. the gas bubbles will migrate back up the siphon downward leg into the air chamber, if the bulk fluid flow velocity is less than the value determined from the equation):
Maximum Allowable Velocity << ![]() ,
,
Where g is 32.2 ft/sec2 and D is siphon inside diameter (ID) in feet.
This equation is an empirical velocity relationship based upon the specific conditions of the test setup and does not take into account bubble size (i.e., length and diameter of bubbles), siphon grades, or the resulting buoyancy of the bubbles, all of which impact bubble transport. For these reasons care should be exercised when utilizing this equation to evaluate the dominance of buoyancy versus bulk fluid flow velocity in the transport direction in the siphon downward leg. It should only be utilized as a guide. [Gibson, 1961; Mathur, 1990; Phifer, et al., 1998; Phifer, et al., 1999a; Phifer, et al., 1999c; Washburn, et al., 1999]
Although the preferred direction of gas bubble transport, with air chamber usage, within the siphon downward leg is upward toward the siphon crest, the siphon with air chamber can function, if the direction of transport is downward and out the siphon discharge. For downward and out gas bubble transport in the downward leg, the bulk fluid flow velocity and not gas buoyancy must dominate transport, so that the gas bubbles are transported down and out the siphon downward leg. This transport is facilitated by higher bulk fluid flow velocities, a continuous downward siphon grade, and the minimization or elimination of fittings, which produce discontinuities in the inside diameter of the siphon line. The grade should be as shallow as possible, to facilitate bulk fluid flow velocity transport downward, and localized high points should be eliminated, so that gas entrapment does not occur. The diameter of the siphon downward leg can be sized to produce the required high, bulk fluid flow velocities (i.e. smaller diameter downward legs will produce higher velocities). However, smaller diameter downward legs in addition to producing higher bulk fluid flow velocities will also create higher head losses and therefore lower flow rates. This is why this is not the preferred direction of gas bubble transport when an air chamber is utilized. [Phifer, et al., 1999a; Phifer, et al., 1999c; Washburn, et al., 1999]
Based upon literature, the following empirical equation may closely represent the bulk fluid velocity, above which bulk fluid flow velocity transport in the siphon downward leg dominates (i.e. the gas bubbles will be transport down the siphon downward leg and out the siphon discharge, if the bulk fluid flow velocity is greater than the value determined from the equation):
Minimum Flushing Velocity > ![]() ,
,
Where g is 32.2 ft/sec2 and D is siphon inside diameter (ID) in feet.
This equation is an empirical velocity relationship based upon the specific conditions of the test setup and does not take into account bubble size (i.e., length and diameter of bubbles), siphon grades, or the resulting buoyancy of the bubbles, all of which impact bubble transport. For these reasons care should be exercised when utilizing this equation to evaluate the dominance of buoyancy versus bulk fluid flow velocity in the transport direction in the siphon downward leg. It should only be utilized as a guide. [Gibson, 1961; Mathur, 1990; Phifer, et al., 1998; Phifer, et al., 1999a; Phifer, et al., 1999c; Washburn, et al., 1999]
An intermediate, bulk fluid velocity exists, where gas bubbles become stagnant, accumulate, and agglomerate within the siphon downward leg. At that point gas bubble transport due to the bulk fluid flow velocity and gas buoyancy are equivalent. However with use of an air chamber the system should not shut itself down. As gas bubbles agglomerate and become larger, the head loss in the siphon increases, which results in a decreased flow rate and a decreased flow velocity. The larger gas bubbles with an increased buoyancy can then overcome the decreased flow velocity and migrate up the siphon downward leg into the air chamber. While the system should not shut down in this situation, the flow rates can be decreased from those calculated based upon single-phase flow assumptions.
3.5 Minimum Flushing Velocity Gas Management
The following are empirical equations used to determine the required minimum flushing velocity (MFV) for the transport of "large" air bubbles out the end of the siphon as determined from laboratory and large-scale tests, respectively, as reported in the literature:
Equation 1) V > ![]() (laboratory-scale empirical equation)
(laboratory-scale empirical equation)
Equation 2) V > ![]() (large-scale empirical equation)
(large-scale empirical equation)
V = velocity in ft/s; g = 32.2 ft/s2; D = internal pipe diameter in ft
These equations show the empirical velocity relationships used to determine the minimum velocity required to transport "large" air bubbles out the end of the siphon. Equation 2 is more conservative than equation 1 and is based upon large-scale testing; therefore its use should be preferred. These relationships are based upon the specific conditions of the test setups and do not take into account bubble size (i.e., length and diameter of bubbles), siphon grades, or the resulting buoyancy of the bubbles, all of which impact bubble transport. For these reasons care should be exercised when utilizing these equations to evaluate the use of MFV for the maintenance of full siphon flow. [Gibson, 1961; Mathur, 1990; Phifer, et al., 1998; Phifer, et al., 1999a; Phifer, et al., 1999c]
In order for MFV to be viable as a method of removing gas from a siphon, line discontinuities and localized high points, which promote bubble accumulation, agglomeration, and potentially entrapment must be eliminated. The following are two potential MFV siphon configurations (see Figure 4) to accomplish this:
Additionally, the inter-related parameters of siphon diameter, bulk fluid flow velocity, siphon grades, and bubble size (i.e. buoyancy) appear to be the parameters, which influence the ability to remove gas from a siphon through the use of MFV. All these parameters and not just bulk fluid flow velocity must be considered when designing a siphon line to maintain full flow through the use of MFV. [Phifer, et al., 1999a]
Gas bubble transport in the upward leg of the siphon occurs toward the crest, due to buoyancy transport in the same direction as bulk fluid flow. This transport is facilitated by higher bulk fluid flow velocities, a continuous upward siphon grade, and the minimization or elimination of fittings, which produce discontinuities in the inside diameter of the siphon. The grade may be shallow, since buoyancy and bulk fluid flow transport is in the same direction, and localized high points should be eliminated, so that gas entrapment does not occur. However the bulk fluid flow velocity in the upward leg is not critical, since buoyancy and bulk fluid flow transport occur in the same direction. Therefore the diameter of the siphon upward leg can be increased so that the bulk fluid flow velocity is less than the MFV so that head loss can be minimized and overall flow rates can be increased. [Phifer, et al., 1999a; Phifer, et al., 1999c]
The direction of gas bubble transport, utilizing MFV for gas management, within the siphon crest and downward leg must be downward and out the siphon discharge. For downward and out gas bubble transport in the crest and downward leg, the MFV or greater must be maintained (i.e. the bulk fluid flow velocity and not gas buoyancy must dominate gas bubble transport). This transport is facilitated by bulk fluid flow velocities greater than MFV, a continuous downward siphon grade, and the minimization or elimination of fittings, which produce discontinuities in the inside diameter of the siphon line. The grade should be as shallow as possible, to facilitate bulk fluid flow velocity transport of gas bubbles downward and out, and localized high points should be eliminated, so that gas entrapment does not occur. The diameter of the siphon downward leg can be sized to produce the required MFV (i.e. smaller diameter downward legs will produce higher velocities). [Phifer, et al., 1999a; Phifer, et al., 1999c; Washburn, et al., 1999]
Bulk fluid flow velocities less than the required MFV result in accumulation, agglomeration, and entrapment of gas bubbles. This increases siphon head loss, which results in decreased flow rates, decreased velocities, and increased gas bubble accumulation, agglomeration, and entrapment. Eventually the system will shut itself down under these conditions.
Even where the MFV mode is viable, its use will often result in slightly lower flow rates than operation with an air chamber. This occurs since smaller diameter siphon lines may be required to obtain the velocities necessary for the MFV mode to transport bubbles out the end of the siphon. Additionally, the MFV mode is not as robust in terms of continuous, consistent, full siphon flow when compared to operation with an air chamber. It relies upon a minimum velocity to sweep bubbles below a certain size out the end of the siphon. Reductions in flow rates, which result in decreased velocities and/or system upsets, that cause larger size bubbles than can be transported, will cause the system to shut down. Such occurrences are difficult if not impossible to predict and can be the result seasonal variations in head, dry conditions, rainfall and infiltration, flooding, etc. The MFV mode should become more robust as the minimum available head to drive the system increases. Additionally the minimization or elimination of fittings, which produce discontinuities in the inside diameter of the siphon, is critical to operation in the MFV mode whereas it is beneficial but not critical to operation in the air chamber mode. For these reasons the air chamber mode of gas management is generally considered preferable over the MFV mode. [Phifer, et. al., 1999c]
4.0 Flow Calculations
Siphon flow rates are typically calculated based upon Bernoulli’s theorem, using a complimentary head loss formula such as Darcy’s formula. These equations are based upon single-phase flow conditions (such as the flow of water only). This method of calculating siphon flow rates will determine the maximum flow that a siphon might produce. However depending upon the siphon configuration, flow rates can range from 50 to 100 percent of that calculated from single-phase flow equations, due to the degassing and transport of gas bubbles within the siphon. This indicates that siphon flow in reality is often two-phase flow (i.e. water and significant gas). Siphons, which minimize the length and maximize the slope (from horizontal) of the siphon downward leg and utilize air chamber gas management, are probably best represented by single-phase flow calculations.
When utilizing siphons in a GeoSiphon™ treatment system, the drawdown or head loss associated with extraction of the groundwater from the aquifer and flow through the treatment media must also be taken into account. These head losses will decrease the differential head available to drive flow through the siphon. Trench extraction systems in general produce less drawdown than well extraction systems. The treatment media geometry should also evaluated versus the minimization of head loss through the media.
[Phifer, et al., 1999a; Phifer, et al., 1999c; Washburn, et al., 1999]
5.0 Summary and Conclusions
GeoSiphon™ systems utilize a siphon to induce contaminated groundwater flow through permeable treatment media. Dissolved gasses within the liquid transported in the siphon will degas due to the vacuum and temperature within the siphon. Appropriate gas management is the primary factor that must be considered for the maintenance of continuous siphon flow, since the accumulation of air within the siphon can stop flow. Typical groundwater de-gassed bubbles consist of the following in descending order: nitrogen, oxygen, carbon dioxide, and argon. Groundwater, which has been treated with granular cast iron, de-gassed bubbles consist of the following in descending order: hydrogen, nitrogen, methane, oxygen, argon, and carbon dioxide.
The following can be done to minimize this degassing:
However degassing within the siphon can not be entirely eliminated therefore one of the following methods of gas management must also be employed: air chamber gas management or minimum flushing velocity (MFV) gas management. An air chamber is essentially a high point accumulation for gas bubbles. The air chamber must be recharged on a periodic basis. This can be accomplished by utilizing a large air chamber that requires manual recharging on an infrequent basis or by utilizing a small air chamber with an automated recharging system. MFV gas management, on the other hand, involves the maintenance of a minimum velocity to transport air bubbles out the end of the siphon. Air chamber gas management is a more robust in terms of continuous, consistent, full siphon flow, since it does not require the maintenance of a minimum velocity as the MFV mode does. Additionally the air chamber mode can often produce greater flow rates than the MFV mode, due to the smaller diameter siphons that may be required to obtain the velocities necessary for the MFV mode. Finally the minimization or elimination of fittings, which produce discontinuities in the inside diameter of the siphon, is critical to operation in the MFV mode but not in the air chamber mode. For these reasons the air chamber mode of gas management is generally considered preferable over the MFV mode.
The empirical velocity relationships, utilized for both the air chamber and MFV modes of gas management, are based upon the specific conditions of the test setups utilized. They do not do not take into account bubble size, siphon grades, the resulting buoyancy of the bubbles, or other parameters which may impact bubble transport. For these reasons these equations should only be utilized with caution as guides.
Additionally, depending upon the siphon configuration, flow rates can range from 50 to 100 percent of that calculated from single-phase flow equations, due to the degassing and transport of gas bubbles within the siphon. Bubble transport within the downward siphon leg particularly affects how the flow deviates from single-phase flow conditions. In reality siphon flow is often a two-phase flow (i.e. water and significant gas). Siphons, which minimize the length and maximize the slope (from horizontal) of the siphon downward leg and utilize air chamber gas management, are probably best represented by single-phase flow calculations.
References
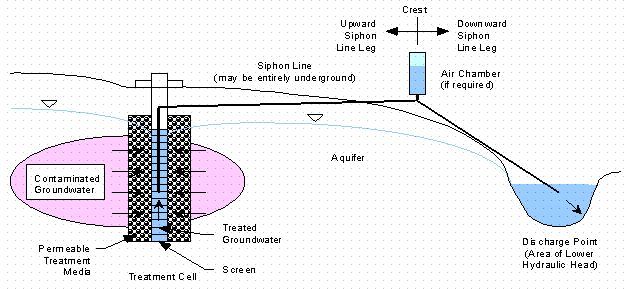
Figure 1. Pre-Siphon Treatment Cell GeoSiphon Configuration
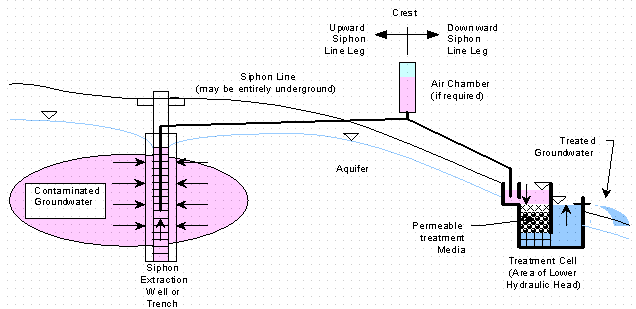
Figure 2. Post-Siphon Treatment Cell GeoSiphon Configuration
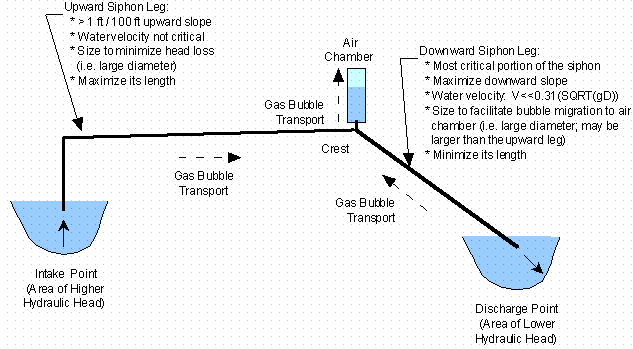
Figure 3. Air Chamber Siphon Gas Management
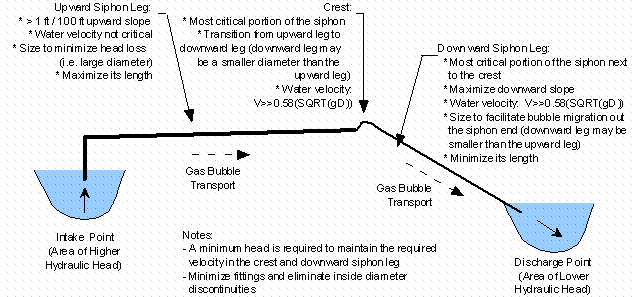
Figure 4. Minimum Flushing Velocity (MFV) Siphon Gas Management