Evaluation of Moisture Limits for Uranium
and Plutonium Mixed Oxides to
Support On-Site Transportation Packaging
R. R. Livingston
Westinghouse Savannah River Company
Aiken, SC 29808
I. Summary
This report contains supporting documentation for on-site shipment of uranium and plutonium mixed oxide materials in the 9975 package. The On-site Safety Assessment (OSA) for transportation of mixed oxide materials from F to H-Area will require implementation of both a percent moisture limit and a total moisture limit for all package contents as defined previously for plutonium residue materials. These limits on moisture content are needed to protect the 9975 package from over pressurization.
Two groups of mixed oxide materials were initially included in the scope of this evaluation of moisture limits. However, evaluation of one group of mixed oxide materials (CZA-104) has been postponed because sufficient background information is not currently available. The second group of mixed oxides (HUA-017 through HUA-020), included in this evaluation has a well know origin and process history. These materials were produced as part of fuel fabrication for the Fast Flux Test Facility (FFTF) and were sent to the Savannah River Site (SRS) when this program was discontinued.
The evaluation of FFTF mixed oxides for transportation in the 9975 package requires an analysis of moisture potentially adsorbed by the mixed oxides after storage at SRS for an extended period of time. Based on the percent moisture and total moisture limits previously established for transportation of plutonium residues:
- The moisture content of FFTF mixed oxides should be sufficiently low to assure that no-net-increase of oxygen gas will be produced during transportation and storage from the radiolysis of adsorbed moisture.
- Sufficient data on FFTF mixed oxides is available to assure that items packaged for on-site transportation will contain less than 75 grams of adsorbed moisture.
Consequently, the results of this evaluation indicate that the FFTF mixed oxides will meet the moisture limits required for use of the 9975 package. To complete this evaluation, without additional laboratory measurements of gas generation, a new limit on decay energy of 2.4 watts per kilogram of mixed oxide mass is necessary. This new limit assures that the radiation dose to adsorbed moisture is bounded by previous Savannah River Technology Center (SRTC) gas generation test data.
The percent moisture limit and a decay energy limit are necessary to maintain the conclusion that mixed oxide materials will produce no-net-increase of oxygen gas during radiolysis of adsorbed moisture. The percent moisture limit for the 9975 was previously set at 2.3% based on a weight loss measurement at 210°C. Because the weight loss measurements at 210°C do not reflect the actual percent moisture contained by mixed oxides, as part of this evaluation, a percent moisture limit of 3.3% is recommended. This new limit is necessary for comparison with published data that are based on laboratory methods that measure total adsorbed moisture. The principle of no-net-increase in oxygen gas is necessary for the OSA evaluation of pressure during a potential hydrogen deflagration.
The total moisture limit of the 9975 package content is set at 75 grams to protect against over pressurization of the containment vessel. The moisture adsorbed by plutonium and uranium mixed oxides can be estimated based on literature reports if the material purity, chemical form and process history are known. The total adsorbed moisture of a mixed oxide material can then be calculated as an alternative to direct laboratory measurement of moisture. This is important because SRS does not currently have a laboratory moisture method applicable to mixed oxide materials.
The evaluation provided in this report can only be extended to materials where the process history and chemical purity have been well established. In cases where little is known about a proposed 9975 package content, the conclusions reached in this evaluation based on "reasonable assumptions" do not apply and will require additional evaluation. The difficulty associated with defining a bounding condition safety basis for on-site transportation, without gas generation testing and analytical measurements, suggests that qualifying more complex mixtures for transportation may require material processing. The process most commonly used to prepare nuclear materials for transportation includes heat treatment to remove moisture and reactive components followed by storage under well controlled humidity conditions to prevent re-hydration.
Alternative means of shipping radioactive materials that do not rely on bounding analysis of gas generation, such as the use of hydrogen getters, should be explored to help alleviate the issues associated with hydrogen gas generation. Implementation of getters for on-site material transportation should reduce the requirements for process knowledge and extensive characterization of the nuclear materials. The benefits resulting from the use of getters will include reducing the need for laboratory measurements and expanding the package’s nuclear material payload and content.
II. Introduction
Use of the 9975 package for transportation of mixed uranium (U) and plutonium (Pu) oxides from F to H-Area requires completion of an On-Site Safety Assessment (OSA). The OSA is required to demonstrate that on-site packaging and transportation provides an equivalent safety basis to implementation of requirements mandated by federal regulations for off-site shipments. This report provides an evaluation of moisture limits for on-site shipping of uranium and plutonium mixed oxides as supporting documentation for the OSA calculations of containment vessel pressure. These pressure calculations are based on prior analysis prepared for shipping plutonium residues from the Rocky Flats Environmental Technology Site (RFETS) to SRS in the 9975 package. This report also contains an evaluation of selected mixed oxide materials for transportation under the F to H-Area mixed oxides OSA.
The technical basis established for shipping RFETS plutonium residues demonstrated that no-net-increase in oxygen gas (O2) occurs during transportation under the specified conditions. In addition, the total pressure of the containment vessel is protected by limiting the total moisture of package content to less than 75 grams (g). These moisture content limits are necessary to assure the package does not exceed the established maximum normal operating pressure (MNOP). By limiting oxygen content in the 9975 package to what is available from the air initially sealed in the package, the consequence (i.e. peak pressure) of a potential hydrogen deflagration or detonation can be calculated as part of the package safety evaluation.
This evaluation of moisture limits for uranium and plutonium mixed oxides is necessary to expand where the principle of no net increase in oxygen gas may be considered a valid argument for on-site transportation. Additionally, due to operating constraints, it is desired to show that mixed oxide materials can meet the OSA moisture requirements without additional analytical moisture measurements of the uranium and plutonium mixed oxides prior to packaging in F-Area.
III. Background
The OSA for shipping mixed oxides from F to H-Area is based on the previous safety analysis developed for shipping of Rocky Flats Environmental Technology Site (RFETS) plutonium residues in the Retrofitted-9975 package. While defining moisture limits for RFETS plutonium residues, gas generation testing was used to demonstrate no-net-increase in oxygen gas content of the container headspace gas is observed when the residue moisture content was limited to 2.3 weight percent (%). The 2.3% weight loss value is based on the change in mass of a sample after heating to 210°C. This analysis technique was selected based on RFETS measurement capability and does not reflect the total moisture adsorbed by plutonium dioxide and other sample matrix components. The 2.3% value is equivalent to approximately 3.3% moisture when comparing to analytical techniques that measure total moisture (e.g. loss on ignition or supercritical fluid extraction) adsorbed on plutonium dioxide samples. Loss on ignition (LOI) measurements in air to estimate total adsorbed moisture are not applicable to mixed oxides due to the weight gain associated with oxidation of uranium dioxide (UO2) to higher oxides when heated in air.
The 2.3% moisture limit for shipping RFETS plutonium residues was established using PuO2 formed by low temperature (~70°C) oxidation of alpha phase plutonium metal having a weapons grade isotopic composition. This oxide is expected to have a surface area ~17m2/g based on a literature report for similar material. Pure PuO2, without added matrix components from the RFETS plutonium residues, was found to provide the most conservative matrix for demonstrating no net oxygen gas generation. The of combination of PuO2 with most residue components led to more rapid reduction in oxygen gas content of the sealed system than observed for PuO2 alone. The exception to this observation is residue materials that contained sodium fluoride (NaF) or sodium bifluoride (NaHF2), which were observed to generate oxygen gas.
The safety analysis for transportation of RFETS plutonium residues required an upper limit of 75 grams total adsorbed moisture in the package. This total moisture limit assures that the maximum normal operating pressure (MNOP) ) associated with payload behavior (chemical or radiochemical reactions that generate hydrogen gas from the adsorbed moisture) does not exceed the 9975’s pressure limit. To evaluate compliance with the 75 grams total adsorbed moisture and 3.3% moisture limits for mixed oxides, without directly measuring the adsorbed moisture content, reasonable assumptions of material purity, processing history, and storage environment must be made.
This report discusses the process and storage necessary to allow mixed uranium and plutonium oxides, with sufficient process history, to meet the requirements for on-site transportation. Based on a review of pertinent literature, the moisture adsorption characteristics of plutonium dioxide (PuO2) have been defined. These same characteristics have historically been applied to UO2 based on the understanding that these refractory oxides behave similarly with regard to moisture adsorption.
The process history, specific surface area, purity, and storage conditions are factors that influence how much moisture is adsorbed by uranium and plutonium oxides. If the mixed oxide materials are similar to PuO2 and have been heat-treated to at least 700°C for an extended time period (e.g. two hours at 700°C will resulting in a specific surface area of ~ 12m2/g), the percent moisture limit should not be exceeded even if the mixed oxide materials are exposed to ambient humidity conditions. However, when a significant fraction of U3O8 or UO3 is present in these materials, the shipper will need to demonstrate that the storage containers have remained sealed from the ambient environment to prevent adsorption of too much moisture.
Demonstrating the mixed oxides meet moisture limits for on-site transportation could be accomplished by first defining the mixed oxides adsorbed moisture when initially packaged and second showing these containers have not gained sufficient weight during storage to exceed the OSA moisture limits. For example, since the FFTF mixed oxides had less than 1% adsorbed moisture prior to storage (based on LOI at 1000°C), any container weight increase during storage may be added the initial moisture content to calculate total and adsorbed moisture based. So, when sufficient records are available, the total adsorbed moisture and percent moisture can be calculated based on the weight change of each storage container and the net weight of the mixed oxide content. Alternatively the moisture concentration of the proposed mixed oxide contents could be measured using an analytical method for total moisture (i.e. Super Critical Fluid Extraction), or gas generation testing of mixed oxides could be used to show these meet the established OSA gas generation requirements.
Based on literature results for adsorbed moisture as a function of surface area and relative humidity, the maximum moisture loading for PuO2 surfaces in milligrams of water per square meter (mg H2O/m2) can be calculated. This value is then used to determine what processing, packaging, and storage condition limits are necessary to prevent exceeding the percent moisture limit and to estimate the total moisture present in a container based on mass of mixed oxide. Because the percent moisture limit for no net oxygen gas generation was determined using PuO2 with a weapons grade isotopic composition, the adsorbed alpha dose or decay energy per unit weight will need to be limited for mixed oxides containing other isotopic compositions of PuO2 (e.g. fuel grade). For example, both fuel and power grade PuO2 have an elevated Pu-238 and Pu-240 isotopic contents, which results in increased alpha decay energy. Other considerations to be examined include the effect of heat treatment prior to shipping to SRS and the potential for subsequent oxidation of UO2 to higher oxides during storage at SRS.
IV. Material Descriptions
The materials intended for shipment to HB-Line for Phase I of the Mixed Scrap Program are described in greater detail by a personal communication from Gary Molen to Kurt Houghtaling. This memo defines two groups of materials that are being considered for shipping under the F to H-Area OSA. The first group is labeled CZA-104 and was produced by burning a metal alloy of natural uranium containing about 0.4% plutonium. Subsequent discussions about the process history of this material have determined that available documentation is currently inadequate to complete an evaluation of moisture adsorbed by this first group of mixed oxides. Consequently, further evaluation of this group of mixed oxides is not included in this report.
The second and larger group of mixed oxides (HUA-017 through 020) is from discontinuance of fuel production for the Fast Flux Test Facility (FFTF). Hanford has provided a significant amount of documentation with this second group of materials, which is now part of the Nuclear Material Stabilization and Storage (NMSS) division’s engineering files.
This group of mixed oxides was produced by blending depleted or natural UO2 with a fuel grade PuO2 powder containing a nominal 12% Pu-240. This second group of materials is labeled HUA-017 through HUA-020 and contains both powders and pellets produced as part of the FFTF program. Each of the HUA batches has a different nominal amount of plutonium oxide ranging from 17 to 25%. These powders were then pressed to form pellets that were subsequently sintered to produce fuel elements. Both powders and pellets are included in the materials stored at SRS. The additional information provided by NMSS relevant to this evaluation are listed here as a summary:
- The mixed uranium and plutonium oxide materials contain greater than 85% actinides based on material assay.
- The uranium content is natural or depleted isotopic composition and is uniformly blended with the plutonium content.
- The plutonium oxide content is less than 30% of the mixed oxide mass and the plutonium has an isotopic composition of less than 15% Pu-240.
- These materials have been in storage at SRS for about 15 to 20 years.
- Current storage of these mixed oxides uses multiple layers of confinement including an outer food can.
- Each container of mixed oxide includes nominally 1.8 kilograms or less of product.
These mixed oxide materials are stored in a variety of container configurations. Frequently materials are placed in metal slip-lid cans, inside sealed plastic bags, and finally stored inside crimp-sealed metal cans. This container configuration is sufficiently tight to prevent the spread of contamination and has been demonstrated to prevent material exposure to potentially humid vault atmospheres. As part of the mixed scrap-processing program, many of these mixed oxide materials will be shipped as currently packaged if sufficient data is available to support the requirements established by the F to H-Area mixed oxides OSA.
V. Moisture Content Limits
Gas generation testing at SRS in early 1999 showed that when the moisture content of PuO2 was limited to 2.3%, based on weight loss at 210°C, no-net-increase in oxygen gas content will be observed in a sealed container during subsequent shipping or storage. The no-net-increase in oxygen was determined by measuring the headspace gas at the end of each test for samples initially packaged in air and containing various amounts of moisture. The headspace gas data were statistically evaluated to demonstrate that samples with 2.3% or less moisture had no more oxygen gas than found in air. Weight loss measurements made for these tests at SRS were done using a thermogravimetric analyzer (TGA), so weight loss was measured as a function of temperature over the range of 30°C up to 900°C. The moisture limit was established using weight loss at 210°C because this matched the RFETS capability for making these measurements. For the F to H-Area mixed oxides OSA, a percent moisture limit is also needed to assure no-net-increase in oxygen gas will occur duringfor on-site shipments.
Technical reports on moisture adsorbed by PuO2 generally express moisture content using loss on ignition (LOI), which is the weight loss associated with heating a sample to a specified temperature (often 900°C to 1000°C) in a low moisture environment. As part of gas generation testing, frequently the moisture contents of PuO2 samples are adjusted by placing each sample in controlled humidity environment and measuring the subsequent moisture adsorption. Weight gain by PuO2 is a function of time, temperature, relative humidity and sample surface area. If the SRS data were expressed as total moisture content, instead of weight loss at 210°C, the moisture limit for no-net-increase in oxygen gas should be set at 3.3%. This 3.3% value was established by evaluation of TGA results and is a conservative estimate of the total moisture adsorbed by samples that indicate 2.3% weight loss at 210°C. Because mixed oxides are expected to behave similarly to PuO2 with regard to moisture adsorption, the 3.3% total moisture limit will be used to evaluate processing and storage conditions to support on-site transportation of mixed oxides without moisture measurements as a prerequisite.
In addition to the percent moisture limit to assure no-net-increase in oxygen gas, there is a 75 gram total moisture limit for the F to H-Area mixed oxides OSA. This moisture control is required to limit the MNOP of the payload based on the assumption that all the adsorbed water is converted to hydrogen gas from either radiolysis or chemical reactions. If all 75 grams of water is converted to hydrogen gas, the theoretical gas pressure inside the 9975 could reach 90% of the vessel design pressure.
VI. Impact of Radiation Dose on Gas Generation
The observation of no-net-increase in oxygen concentration is expected to result from chemical reactions that consume oxygen gas and oxygen-containing free radicals produced from the radiolysis of water. Oxygen gas is not a primary product from the radiolysis of water, but results from decomposition of intermediates such as hydrogen peroxide. In order for oxygen gas to be generated from the radiolysis of moisture, Wagh et. al. stated that oxygen-containing radicals would have to recombine faster than they react with the available material surfaces. Many other examples of oxygen consumption during storage of radioactive materials are known. These include radioactive materials encapsulated in cement and storage of transuranic (TRU) wastes. When headspace gases have been sampled in cans of PuO2 or plutonium residue in storage, the results of these measurements also have indicated a depletion of the oxygen gas. However, oxygen generation has been confirmed in multiple instances associated with the storage of radioactive sludges which presumably have a high moisture content and other components not studied as part of this evaluation.
Conditions that would favor production of radicals faster than they can be scavenged by the matrix material surfaces are anticipated as the moisture content of a sample increases. In the SRS gas generation study, the effect of moisture concentration on O2 gas generation samples of PuO2 and RFETS plutonium residues was evaluated. The net increase in oxygen gas was observed only with pure PuO2 samples when water was added to the sample, in excess of what could be adsorbed upon extended exposure to 100% relative humidity. These tests were conducted using PuO2 resulting from low temperature corrosion of alpha-phase plutonium metal having weapons grade isotopic composition. Other sources of PuO2, that have a much higher surface area (e.g. PuO2 produced from calcination of plutonium oxalate at 400°C has a surface area of ~30m2/g), are known to adsorb greater amounts of water. These higher surface area oxides may adsorb sufficient moisture to exceed the 3.3% limit established for no net generation of oxygen gas. When other matrix components were present, or the temperature was elevated, oxygen gas content was observed to decrease over the course of each test. The only exceptions to this observation have been noted earlier in this report and are anticipated to be the result of chemical reactions, not radiolysis of adsorbed moisture.
The alpha energy, produced by radioactive decay of the plutonium isotopes, generates oxygen-containing radical species, which are described in detail by Wagh et. al. As the sum of decay energies per unit mass of PuO2 increases, the percent moisture limit established to prevent accumulation of oxygen gas is expected to drop. No known experiments have been conducted with mixed oxides to measure the impact of increased decay energy on the potential for a net increase of oxygen gas of a sealed container. Therefore, the decay energy (watts, or W) per kilogram (kg) net weight for mixed oxide shipments should be controlled to less than or equal to the decay energy per kilogram of PuO2 used to establish the initial percent moisture limit.
The 3013-99 Storage Standard includes a table of decay energies for the various isotopic mixtures commonly encountered at Department of Energy (DOE) facilities. The initial energy output – prior to Am-241 in-growth – is the minimum decay energy. During the course of isotope decay, these materials reach maximum decay energy. Both the initial and maximum decay energies are provided by the 3013-99 standard. This data is shown for both Weapon Grade and Fuel Grade materials in Table I. In a similar fashion, the decay energy for PuO2 used in the SRS gas generation testing and a worst case value for FFTF mixed oxides have been calculated and are shown in Table I.
Table I. Energy Output of Pu Plutonium Isotopic Mixes
|
Nuclide |
Weapon Grade |
Fuel Grade (from 3013) |
SRS Gas Test |
FFTF |
|
Pu-238 |
0.05% |
0.1% |
0.013% |
0.13% |
|
Pu-239 |
93.50% |
86.1% |
94.39% |
84.96% |
|
Pu-240 |
6.00% |
12.0% |
5.42% |
12.48% |
|
Pu-241 |
0.40% |
1.6% |
0.16% |
0.95% |
|
Pu-242 |
0.05% |
0.2% |
0.20% |
0.35% |
|
Am-241 |
- |
- |
0.11% |
1.55% |
|
Initial watts/kg |
2.53 W/kg |
3.15 W/kg |
2.28 W/kg |
3.67 W/kg |
|
Maximum watts/kg |
2.81 W/kg |
4.48 W/kg |
2.40 W/kg |
5.17 W/kg |
The ratio of decay energy for the SRS PuO2 to the decay energy for a "worst case" FFTF PuO2, which has been aged about 20 years, yields a factor of approximately 2.2 (5.17 ¸ 2.40 = 2.15). "Worst case" has been defined as the PuO2 containing the maximum or upper specification limit for Pu-238, Pu-240 and Pu-241, where the decay of Pu-241 (with a 14.4 year half-life) to Am-241 (which generates 114.8 W/kg) results in a significant increase of decay energy (from 3.7 to 5.2 W/kg in ~20 years). So the PuO2 component of the FFTF mixed oxide will have about 2.2 times the decay energy of the SRS PuO2 used in gas generation testing. To maintain the decay energy per gram of mixed oxide at a level not to exceed 2.4 W/kg, the "worst case" FFTF PuO2 must be sufficiently diluted with uranium. Therefore, the PuO2 content of FFTF mixed oxide could be as high as 46% without exceeding the 2.4 W/kg limit (i.e. 2.4÷5.2 x 100% = 46.2%).
The decay energies for plutonium isotopic compositions conservatively bound the decay energies associated with depleted and natural uranium isotopic compositions that are used to form mixed oxide fuels. So, the decay energy associated with the uranium content (i.e. the energy output of U-235 and U-238) found in FFTF mixed oxides is negligible. Because the PuO2 content of the FFTF materials currently stored at SRS is less than 30%, the requirement to limit decay energy per kilogram net weight does not exclude any FFTF materials for on-site transportation. Similar calculations should be performed for other mixed oxide compositions that may need to be packaged for on-site transportation as part of the F to H-Area mixed oxides OSA. These calculations could easily be completed using data from Tables B-5 and B-6 in 3013-99 DOE Standard. Because the F to H-Area mixed oxides OSA limits the package content to 3 watts, only about 1.9 kg of a FFTF mixed oxide containing 30% "worst case" PuO2 (i.e. 3 watts / (0.30 x 5.2 W/kg) = 1.92 kg) would be permitted in the vessel. As the percentage of PuO2 in the FFTF mixed oxide decreases, the amount of mixed oxide allowed in the vessel increases proportionally.
VII. Adsorption of Moisture on Mixed Oxides
The NUMEC P-90 and P-100 reports suggest that moisture is adsorbed on refractory plutonium and uranium oxides in a similar fashion. Based on a review of literature for moisture adsorption on PuO2, the parameters of importance, in estimating the moisture adsorbed by mixed uranium and plutonium oxides, include the oxide’s specific surface area, moisture content in the air or relative humidity, and the source or preparation method for the oxides. Specific surface area (m2/g) is controlled in part by the method of preparation and ranges from about 1m2/g up to 60m2/g for PuO2. Heating at elevated temperature can further reduce the surface area. For example, PuO2 formed by oxalate precipitation and calcination for about 15 minutes at 350°C to 400°C will have a surface area of about 60m2/g, but further heating of this material to 760°C reduces the surface area to about 10m2/g. The length of time a PuO2 sample is heated also effects the surface area obtained by heating to an elevated temperature. For example, the surface area of a freshly prepared PuO2 sample heated to 760°C is reduced linearly during the first 30 minutes of heating. Specific surface area was shown to decreases from 10.8 m2/g at 5 minutes to 7.5 m2/g at 17 minutes to 4.5 m2/g at 30 minutes. The amount of moisture that can be adsorbed by PuO2 increases with larger surface areas and higher relative humidity.
A. Calculation of Maximum Adsorbed Moisture on PuO2
Although moisture adsorption on uranium and plutonium oxides has been a topic of discussion for many years, only a few published measurements have been identified. There are several variables that impact the adsorption of moisture on PuO2; however, the capacity of PuO2 to adsorb moisture is proportional to the PuO2 surface area. Table II has been assembled from the known test data and is used to calculate the maximum moisture expected to be adsorbed on PuO2 from humid air in milligrams of water per square meter of PuO2 surface area (mg H2O/m2).
The mass of water per square meter (mg H2O/m2), presented in the fourth column of Table II, is calculated by dividing the weight gain measurements (mg H2O/g PuO2) by the specific surface area (m2/g). The number of adsorbed water layers is calculated by dividing the mass of water per square meter by the theoretical mass of water per square meter of surface area. The theoretical value used in developing this table is 0.213 mg H2O/m2/layer. This theoretical value has been determined by calculating the spacing of water molecules required for uniform single molecular layer of water coverage on a PuO2 surface.
The data points in Table II from SRS moisture adsorption tests were collected after about 20 days of exposure to the listed humid environment. The conditions for many of the other tests described were completed using short term moisture exposures; however, nearly identical values for mg H2O/m2 were found by other researchers presented in Table II.
Table II. Moisture Adsorbed by PuO2 as Function of Surface Area
|
Row |
Surface Area |
Relative Humidity (%) |
Water Adsorbed (mg/g) |
Moisture Content |
Molecular Layers of Water |
Comments about Test |
References |
|
1 |
1.3 |
76 |
0.95 |
0.73 |
3.3 |
16 |
|
|
2 |
1.3 |
0 |
0.86 |
0.66 |
3.1 |
7 cycles, 0 to 100% RH |
20 |
|
3 |
1.3 |
45 |
1.3 |
1.0 |
4.5 |
7 cycles |
20 |
|
4 |
1.3 |
76 |
1.7 |
1.3 |
5.9 |
7 cycles |
20 |
|
5 |
57 |
97 |
35 |
0.61 |
2.9 |
Data from P-80 and P-90 |
17 |
|
6 |
57 |
76 |
19 |
0.33 |
1.5 |
P-80/P-90 |
21 |
|
7 |
57 |
55 |
13 |
0.22 |
1.0 |
P-80/P-90 |
21 |
|
8 |
53 |
97 |
26 |
0.49 |
2.3 |
P-80/P-90 |
21 |
|
9 |
53 |
76 |
14 |
0.26 |
1.2 |
P-80/P-90 |
21 |
|
10 |
53 |
55 |
10 |
0.19 |
0.9 |
P-80/P-90 |
21 |
|
11 |
9.9 |
97 |
18 |
1.8 |
8.5 |
P-80/P-90 |
21 |
|
12 |
9.9 |
76 |
6 |
0.61 |
2.9 |
P-80/P-90 |
21 |
|
13 |
9.9 |
55 |
3 |
0.30 |
1.4 |
P-80/P-90 |
21 |
|
14 |
17* |
76 |
22 |
1.3 |
6.1 |
SRS, long exposure |
2 |
|
15 |
17* |
100 |
29 |
1.7 |
8.0 |
SRS, long exposure |
2 |
|
16 |
17* |
Added H2O |
33 |
1.9 |
8.9 |
moisture |
2 |
|
17 |
20 |
>95 |
35 |
1.8 |
8.2 |
18 |
|
|
18 |
17 |
100 |
28.5 |
1.7 |
7.9 |
20 |
* Surface area for these samples is estimated
base on measured surface area of materials prepared
in a similar fashion (reference 23).
The SRS values of 1.3 and 1.7 mg H2O/m2 at 76% and 100% relative humidity (RH) may not have reached equilibrium moisture content and are subsequently short of the expected 10 molecular layers. However, these results are reasonably close to the estimate of 2.1 mg H2O/m2 expected for PuO2 on exposure to 100% relative humidity. In this same publication Haschke and Ricketts estimate the number of adsorbed water layers expected for PuO2 as a function of relative humidity. These values are reproduced in Table III.
Using the estimates from Table III, for the number of molecular layers expected from exposure at 76% and 100% relative humidity, Table IV is presented to provide a comparison between observed moisture adsorption data from Table II and the theoretical estimate of adsorbed moisture. The number of theoretical water layers adsorbed for each relative humidity level in Table III was estimated by assuming the 6 layers adsorbed between 1% and 80% relative humidity are adsorbed in a linear fashion. So, an additional layer of moisture is added about every 13% relative humidity (e.g. 1-14% has 2 layers, 15-28% has 3 layers, 29-42 has 4 layers, 43-56 has 5 layers, 57-70 has 6 layers, and 70-83 has 7 layers).
Table III. Sequential Types of Water Adsorption on PuO2 at Room Temperature
|
Sequential Type of Adsorption |
Number of molecular layers |
Relative Humidity Required (%) |
Nature of Sorption |
|
1 |
0.5 |
<<1 |
Strong chemisorption |
|
2
|
0.5 |
<1 |
Chemisorption |
|
3 |
6 |
>5 |
Strong physisorption |
|
4 |
2 |
>80 |
Moderate physisorption |
|
5 |
1 |
>95 |
Weak physisorption |
Table IV. Calculated Moisture Adsorbed on PuO2 at 76% and 100% Relative Humidity
|
Relative Humidity (%) |
Estimated
|
Surface Area (m2/g) |
Calculated H2O (mg/g) |
Observed value (mg H2O /g) |
Table II |
|
45 |
5 |
1.3 |
1.4 |
1.3 |
3 |
|
55 |
5 |
9.9 |
7.1 |
3 |
13 |
|
55 |
5 |
53 |
56 |
10 |
10 |
|
55 |
5 |
57 |
60 |
13 |
7 |
|
76 |
7 |
1.3 |
1.9 |
1.7 |
4 |
|
76 |
7 |
9.9 |
14.5 |
6 |
12 |
|
76 |
7 |
17 |
25 |
22 |
14 |
|
76 |
7 |
53 |
78 |
14 |
9 |
|
76 |
7 |
57 |
84 |
19 |
6 |
|
>95 |
10 |
20 |
42 |
35 |
17 |
|
97 |
10 |
9.9 |
21 |
18 |
11 |
|
97 |
10 |
53 |
111 |
26 |
8 |
|
97 |
10 |
57 |
120 |
35 |
5 |
|
100 |
10 |
17 |
36 |
29 |
15 |
|
100 |
10 |
17 |
36 |
28.5 |
18 |
In general it is seen from Table IV that the published results are in good agreement with, but lower than, the theoretical estimate. However, the theoretical estimate is off by a factor of about five for the higher surface area (i.e. both 53 and 57m2/g) PuO2 materials tested by NUMEC. Never the less, using this theoretical approach to estimate adsorbed moisture will lead to conservative estimates of the percent and total moisture adsorbed by mixed oxides. For most materials currently in storage, the specific surface area has not been measured and must be estimated based on process history. So in addition to the relative humidity conditions for the storage environment, an estimate of the material’s specific surface area also is needed to support the calculation of adsorbed moisture.
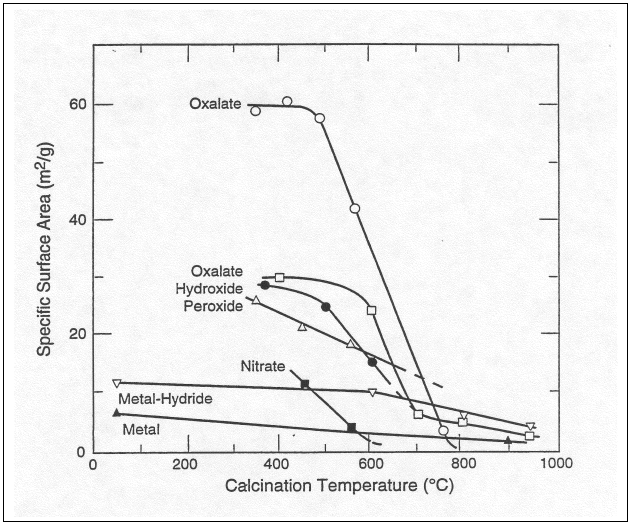
The effect of calcination temperature on PuO2 surface area from various sources is reproduced from Haschke and Ricketts in Attachment I. Because PuO2 from oxalate precipitation tends to have the highest surface area, data for mixed oxide from this source will be used to provide conservative estimates of specific surface area as a function of calcination temperature. It is well documented that the specific surface area of PuO2 samples decrease upon heating above about 400°C. The time a sample is held at the calcination temperature also impacts the measured surface area. This relationship was clearly demonstrated by heating a sample of PuO2 to 760°C for hold times of 5 to 30 minutes. The results of this test showed a linear decrease in specific surface area of about 50% (from 11 down to 5 m2/g).
Two sets of specific surface area data are known for PuO2 produced from oxalate. However, these two sets of data vary dramatically in the length of time samples were held at the calcination temperature – from a nominal 15 minutes up to 12 hours. Samples heated for two hours, like the FFTF mixed oxides, are anticipated to have surfaces areas more like the materials heated for 10 hours because much of the surface area reduction is observed in the first 30 minutes of heating. Table V is produced using curve fits of calcination temperature and specific surface area from references 21 and 15 (Set 1 and Set 2 respectively) for temperature values above 450°C. The data for set 1 represents the PuO2 that was heated for a nominal 15 minutes, and set 2 represents the PuO2 that was heated for approximately 10 hours.
Table V. Specific Surface Area for PuO2 Produced from Plutonium Oxalate
|
Calcination |
Set 1 |
Set 2 |
|
640 |
32 |
18 |
|
700 |
22 |
12 |
|
760 |
12 |
8 |
By knowing the source and temperature history of a mixed oxide material, the specific surface area can be estimated from published data. Table VI presents a conservative estimate of percent moisture based on estimated surface area (from Table V) and theoretical moisture capacity (from Table III and Table IV). Based on Table VI, all PuO2 materials from oxalate that have been processed at 700°C or higher for an extended period of time (e.g. two hours) are expected to achieve a sufficiently low surface area to prevent adsorption of more than 2.6% moisture. Since the FFTF mixed oxides being considered for Phase I of the HB-Line Mixed Scrap Campaign have been heated to 700°C for two hours, these should be unable to adsorb moisture in excess of the 3.3% moisture limit established to prevent a net increase in oxygen gas. In reaching this conclusion, it is important to recognize that the uranium oxide species present must have moisture adsorption characteristics similar to PuO2. Further discussion of this issue is provided in section VIII C, Adsorption of Moisture on UO2, U3O8 and UO3.
Table VI. Percent Moisture Estimates for PuO2 Based on Estimated SSA and Theoretical H2O Layer
|
Relative Humidity |
H2O |
Oxalate Calcination |
Surface |
Calculated Moisture Content (%) |
Comments
|
|
76 |
7 |
640 |
18 |
2.7 |
* Using data from Set 2 in Table V. |
|
100 |
10 |
640 |
18 |
3.8 |
|
|
76 |
7 |
700 |
12 |
1.8 |
|
|
100 |
10 |
700 |
12 |
2.6 |
|
|
76 |
7 |
760 |
8 |
1.2 |
|
|
100 |
10 |
760 |
8 |
1.7 |
B. Layers of Water on PuO2
One theory supporting the production of oxygen gas during radiolysis of water postulates the actual hydrogen gas generation rate depends on how tightly the adsorbed water is bound on the PuO2 surface. As the number of water layers increases, the binding forces decrease, allowing a more rapid production of hydrogen gas. Once the binding forces are sufficiently small, both hydrogen and oxygen gas are generated, as in aqueous solution. Although high surface area PuO2 samples are expected to adsorb more moisture from humid air than a low surface area counterpart, the number of water layers for various surface area samples is expected to remain constant for a given humidity environment. So the higher percent moisture adsorbed on a high surface area PuO2 sample from humid air many not lead to an increase in the oxygen gas content of a sealed container. In the SRS tests at 100% relative humidity, only eight molecular layers of water were calculated to actually adsorb during about 20 days (Table II, row 15). Haschke and Ricketts report that about ten layers are generally adsorbed during exposure of PuO2 to 100% relative humidity. The SRS data use an estimate of surface area (17m2/g) based on the observation of Stakebake for PuO2 produced in a similar fashion. Decreasing this surface area estimate by a small amount would increase the number of calculated layers to provide layer values that match the theoretical limit of ten proposed by Haschke and Ricketts. However, none of the other data points presented in Table II reached this theoretical limit either, suggesting perhaps that the actual limit of molecular layers is actually less than ten.
The percent moisture limit set based on SRS gas generation testing is 3.3% (Table II, row 16) which results in a calculated 8.9 molecular layers of moisture. From Table II, it can be observed that none of the other various samples stored in humid environments was able to adsorb more than about 8.5 layers of water or about 2.9% moisture. The sample containing 3.3% moisture was created by direct addition of liquid water to the PuO2 sample. This observed, limited ability of PuO2 to adsorb moisture from humid environments may be a characteristic of pure PuO2 or mixed oxides that can be used in future shipping efforts.
Additional testing would be needed to confirm that the number of water layers actually controls the hydrogen gas generation rate and the no net increase in oxygen gas observation. This testing would need to be conducted using a single source of PuO2 that has been heat-treated to change the surface area. Measuring the surface area of these samples followed by adsorption of moisture and gas generation testing would help identify the role of surface area and layers of water on gas generation in PuO2 materials. Because the isotopic composition of PuO2 is not expected to impact how much moisture can be adsorbed, the effect of increasing decay energy on gas generation also would need to be tested. This test would use PuO2 produced from various isotopic compositions to determine if the moisture limit for no-net-increase in oxygen gas changes as a function of decay energy.
C. Adsorption of Moisture on UO2, U3O8 and UO3
The PuO2 compound is the highest oxidation state traditionally accepted by actinide chemists; however, UO2 can be further oxidized with a number of intermediate steps to U3O8 and on to UO3. The further oxidation of UO2 is important to this evaluation because each of these uranium compounds adsorbs moisture differently. The kinetics for conversion of UO2 to higher oxidation states is controlled by a number of parameters including radiation dose, oxygen availability, moisture concentration and temperature. Because rapid oxidation of UO2 begins at about 185°C, any heat treatment of a UO2/PuO2 mixed oxide materials in air will accelerate the conversion of UO2 to U3O8.
The oxidation rate of mixed oxides in dry air is greatly retarded as the PuO2 content increases. However, the radiation dose associated with an increased PuO2 content will increase the oxidation rate of uranium oxides largely from the production of NOx that is formed by the radiolysis of moist air. The presence of NOx greatly accelerates oxidation of UO2 and leads to the formation of UO3 even at low temperature. Katz and Rabinowitch report that UO2 reacts rapidly and exothermically with NO2 even at low temperatures. In the presence of greater than 40% relative humidity, oxidation of UO2 produces both U3O8 and UO3∙xH2O where x is between 0.7 and 0.9. The conversion of UO2 to U3O8 is accompanied by a 36% expansion in volume that can cause mixed oxide pellets to crumble and expose additional surface for potential moisture adsorption.
Recent unpublished data from ORNL show that uranium oxides with a 0.5m2/g surface area, when exposed to 97% relative humidity, adsorb moisture very differently as the O/U ratio increases. For example, UO2 did not adsorb any measurable quantity of moisture, while U3O8 adsorbed 1.6% moisture in 16 days and appeared to be increasing in weight at a constant rate. The ORNL observations for UO3 showed a 23% weight gain, which is in excess of the water required to form UO3·2H2O. No published data on moisture adsorption by U3O8 and UO3 have been identified at this time for various conditions of moisture, surface area, temperature, etc. However, based on these recent moisture adsorption results from ORNL, mixed oxides containing U3O8 and UO3 may potentially adsorb sufficient moisture to exceed the OSA limits for percent moisture and the total moisture.
The UO2 used to produce the mixed oxide can be expected to have a surface area that is initially less than 6 m2/g. There are two clear reasons for this assertion:
- Extensive data on surface area for UO2 for various production methods consistently show surface areas less than 60m2/cc with a density of 10 to 11g/cc. (i.e. 60m2/cc divided by 11g/cc is less than 6 m2/g)
- More recently investigators have shown that UO2 with a surface area greater than 8.4m2/g is pyrophoric in air.
When UO2 has been converted to higher oxides as described by the foregoing mechanisms, the moisture adsorption characteristics can no longer be described as similar to PuO2 as presented in the NUMEC P-90 Report and a more recent LANL review. This low initial surface area is important to minimizing the moisture adsorbed, like observed for PuO2.
Askew has recently completed calculations based on literature reports, which demonstrate that UO2 is oxidized slowly even in air at ambient temperature. The oxidation rate of UO2 to U3O8 occurs in steps with an intermediate composition of U3O7. The conversion of UO2 to U3O7 is dependent on both temperature and particle diameter because the reaction rate is diffusion limited. After 15 years of storage in air at 50°C, less than 5% of 10 micron diameter particles are estimated be converted to U307, while about 40% of 1 micron diameter particles would undergo conversion. The particle diameter of FFTF mixed oxide components is not known; however, the typical particle diameter for similar materials was reported to range from 1 to 17 microns and varied principally with method of preparation. Clearly if mixed oxides are left exposed to air, a substantial amount of UO2 could be oxidized during long term storage.
A review of shipping records for the FFTF mixed oxides, which includes the material and process description, shows that the initial step for preparing the mixed oxide was ball milling UO2 and PuO2 powders in a dry nitrogen environment for 20 hours. Heat treatment of process scrap powders was done at 700°C for two hours in an argon-8% hydrogen mixture, which prevents oxidation of the UO2. Mixed oxide pellets that may contain an organic binder were heat-treated at 1000°C under similar conditions. These FFTF mixed oxide materials were subsequently packaged in dry nitrogen. Storage of the mixed oxide materials in sealed containers at moderate temperatures would retard the oxidation of UO2 and subsequent adsorption of moisture from the ambient environment.
Based on an assumption that the storage containers have remained sealed, the uranium oxide content of FFTF mixed oxides should remain primarily UO2 and adsorb water similar to PuO2. This assumption can be readily tested when the initial weight of each container is known prior to storage and current container weight measurements can be made because the oxidation of UO2 to U3O8 and moisture adsorption both contribute to each container’s weight. Engineering records show that the FFTF materials sampled prior to sealing in the produce cans had less than 1% LOI at 1000°C. When containers of FFTF mixed oxide can be demonstrated to have not gained weight, these would still have less than 1% adsorbed moisture. If the produce cans have gained weight during storage, the weight gain for items to be shipped under the F to H-Area mixed oxides OSA should not exceed 2.3% of the mixed oxide net weight (i.e. 1% initial + 2.3% gained = 3.3% total). If the mixed oxide net weight is not available, a stable container gross weight (i.e. no weight gain during storage) still demonstrates the produce can did not leak, and the UO2 has not oxidized or adsorbed moisture.
It is reasonable to expect that these produce cans have not leaked because surveillance activities in the FB-Line vaults have shown that similar containers, with items that are chemically reactive toward air (e.g. plutonium metal), have not gained weight over many years. If these chemically reactive materials were exposed to air, the weight of these containers would increase as the materials reacted with air and the weight gained during the past 15 to 20 would be easily observed as part of the surveillance program. These containers were also packaged at Hanford then shipped to FB-Line in a fashion similar to FFTF mixed oxides in the early to mid 1980s.
VIII. Discussion of Total Moisture Limit
Assuming the mixed oxides reflect the moisture adsorption characteristics of PuO2, processing conditions can be established to control the respective items’ moisture content. This is important because items that have not adsorbed moisture during storage, but need to be repackaged prior to shipment, will adsorb moisture if not handled in a dry atmosphere. The amount of moisture that can be adsorbed by mixed oxides during exposure to humid environments was discussed in section VII, Adsorption of Moisture on Mixed Oxides. This data can be used to estimate the amount of mixed oxide that can be packaged for transportation under the F to H-Area mixed oxides OSA.
As described previously, the F to H-Area mixed oxides OSA imposes a 75 gram limit on total moisture. Using the percent moisture limit of 0.033g H2O/g bulk mixed oxide (3.3%), a mass limit of approximately 2270 grams of bulk mixed oxide is calculated (i.e. 75g H2O ÷ 0.033g H2O/g bulk = 2272.7g bulk), and could be used to simplify packaging operations. To increase this allowed bulk loading, this calculation can be completed using actual adsorbed percent moisture or a more appropriate value from Table VI (i.e. 1.8% moisture for materials calcined at 700°C and exposed to 76% relative humididy). If the FFTF mixed oxide containing 30% "worst case" PuO2 is being packaged, this materials is limited to about 1.9 kilograms of bulk weight because the F to H-Area mixed oxides OSA wattage limit (i.e. 3W÷ (0.30 x 5.2 W/kg) = 1.9 kg). Alternatively, the heat generated by each container may be calculated based on an actual PuO2 fraction and isotopic composition of the mixed oxide payload.
If produce cans that have not leaked are repackaged in a very dry environment (i.e. –17°C dew point), these materials should not adsorb additional moisture during repackaging. Consequently, the total adsorbed moisture should not increase and could be packaged for transportation up to the 4.4 kilogram radionuclide mass limit established for the 9975 (i.e. 75g H2O ÷ 0.01g H2O/g bulk = 7500g bulk). Where weight gains have been noted for the produce cans of FFTF mixed oxide in storage, the percent moisture of these mixed oxides can be calculated based on the produce can weight gain and the mixed oxide net weight. Using this data, the amount of mixed oxide allowed under the 75 gram total moisture limit can be calculated assuming that all the weight gain is associated with the adsorption of moisture by the mixed oxides. These produce cans should also be repackaged in a very dry environment.
IX. Headspace Gas Measurement for Mixed Oxides in Storage
Two documents providing headspace gas concentration data on mixed oxide materials, similar to items in storage at SRS, are known at this time. Neither of these items generated a significant quantity of oxygen gas. In the Los Alamos National Laboratory (LANL) Materials Identification and Surveillance (MIS) program, a can or of mixed oxide material nearly identical to the materials at SRS was sampled for headspace gas analysis. This can held about 740 grams of mixed oxide with 15% fuel grade plutonium. The moisture content of this material was about 0.15% and x-ray diffraction analysis revealed a significant quantity of U3O8. The cans used to package this sample were shown to not leak. The gas sample showed that the oxygen gas had been consumed to 0.1% from an estimated starting concentration of 20.9% and the hydrogen gas concentration was up to only 0.3% after more than ten years in storage.
The Atomic Energy Research Establishment (AERE) samples were mixtures of PuO2 and U3O8 that had been canned after 2.5 years of storage in a PVC bag. After about six months in storage, two cans were identified as pressurized. The headspace gases from these cans were sampled and analyzed prior to heating the contents in vacuum at 150°C for repackaging. The gas composition included large amounts of hydrogen and methane gas (CH4) but only trace quantities of oxygen gas (ND or 0.05%). The initial packaging gas was argon. The moisture content of these samples was not reported, but the heat/vacuum treatment was sufficient to prevent pressurization after repackaging. The methane content of this sample was attributed to residual carbon remaining from the plutonium carbide source material used to generate this material.
X. Potential Gas Generation RateThis section is provided to underscore the potential impact of packaging materials that have high moisture contents. The potential of these materials to exceed a flammable concentration of hydrogen gas has been previously demonstrated. The results of calculations shown below are an extrapolation of short-term test data to a much longer time period and the effects of plutonium isotopic composition and material purity have not been considered in this estimate. Increases in decay energy associated with some isotopic compositions are expected to generate hydrogen gas at a more rapid rate; however, the presence of impurities (even UO2) and elevated temperature are expected to contribute to a more rapid decrease in oxygen gas.
Based on experience from the gas generation results for PuO2, the intent of this calculation is to show that if a large amount of adsorbed moisture is present, significant quantities of hydrogen gas could be generated in the sealed system. The amount of gas generated by 1800 grams of mixed oxide material containing 25% PuO2 at the 3.3% moisture limit would be about 10 standard cubic centimeters (cc) per day. Assuming a package void volume of 2000 cc, the hydrogen gas concentration would exceed 4 volume percent (the lower flammable limit for hydrogen gas in air) in about eight days, and the package could exceed 2.5 atmospheres pressure at the end of one year. These data are calculated based on prior gas generation test rate measurements for PuO2, which showed that about one micromole (mmol) of hydrogen gas generated per gram of plutonium per day (1 mmol/g Pu/day) is generated by a sample at the 3.3% moisture limit.
XI. Recommendation for Future On-Site Shipping
The difficulty associated with estimating the impact of gas generation within transportation packages will increase, as the material content becomes less certain. To deal effectively with the wide range of materials potentially requiring shipment, the implementation of getters or recombiners for on-site shipping should be considered. By using getters or recombiners in on-site shipping, the concerns that relate to hydrogen deflagration or detonation during transport may be alleviated. Controlling hydrogen accumulation versus generation would appear to have greater reliability and be less costly. Without controlling hydrogen generated within the shipping containers, shipping additional on-site materials will require material repackaging, chemical analysis, and potentially heat treatment or gas generation testing.
XII. Conclusions
The prior gas generation technical basis for shipping plutonium residues required that no-net-increase in oxygen gas occurred during transportation and that the total moisture content of the package be less than 75 grams. These same controls are applicable to shipping mixed oxide materials containing predominantly PuO2 and UO2. The requirement for no-net-increase in oxygen gas will be met for mixed oxides when the percent moisture content is less than 3.3% and the decay energy is less than 2.4 watts per kilogram. This new limit on decay energy is necessary to allow comparison with previous gas generation studies that established the 3.3% moisture limit for no-net-increase in oxygen gas. Consequently, the controls necessary for packaging mixed oxides in the 9975 to meet the F to H-Area OSA requirements for no-net-increase in oxygen gas are as follows:
- adsorbed moisture content £ 3.3%
- decay energy £ 2.4 watts/kilogram
Because a significant fraction of U3O8 may be present in these materials when the storage container leaks, the shipper will need to evaluate the weight gained during storage of the mixed oxides to assure the moisture content remains less than 3.3%. Moisture measurements for total adsorbed moisture content of the mixed oxides will also satisfy the operating constraints required for implementation of the OSA. Additional research related to the moisture adsorbed by U3O8 and additional gas generation testing may support easing these restrictions in future revisions of the OSA.
In cases where the materials’ process history or purity is not known, the conclusions reached in this evaluation based on "reasonable assumptions" do not apply and will require a separate evaluation. Future evaluations of this type will become increasingly difficult to complete as the package contents become more complex and knowledge of the contents is less certain. For this reason, it is necessary for SRS to begin looking for alternatives that offer improvements for transportation of materials that generate hydrogen gas within the package.
Attachment 1. Effect of Oxidation
Temperature on PuO2 Surface Area
(Reproduced from Reference 15, Figure 1,
p. 27)
References
- WSRC-RP-99-00659, Use of the Retrofitted 9975 Shipping Packaging to Ship Sand, Slag, and Crucible Materials, Justification for Continued Use, Westinghouse Savannah River Company, Aiken, SC, August 1999.
- WSRC-TR-99-00223, Gas Generation Test Support for Transportation and Storage of Plutonium Residue Materials, R. Livingston, Savannah River Technology Center, August 1999.
- NUMEC P-90, Progress Report for Development of Plutonium-Bearing Fuel Materials, Nuclear Materials and Equipment Corporation, Apollo, Pennsylvania, December 1961.
- NUMEC P-100, Progress Report for Development of Plutonium-Bearing Fuel Materials, Nuclear Materials and Equipment Corporation, Apollo, Pennsylvania, March 1962.
- LA-UR-99-261, Hydration of Plutonium Oxide and Process Salts, NaCl, KCl, CaCl2, MgCl2: Effect of Calcination on Residual Water and Rehydration - Revision 2, D.M. Smith, M.P. Neu, and L.A. Morales, Los Alamos National Laboratory, January 2000.
- Personal Communication, Gary Molen to Kurt Houghtaling, Subject: Revised Contents Tables for F to H Shipments - 9975 OSA, December 1999.
- WSRC-TR-99-00236, Thermal Analysis of RFETS SS&C, P.S. Korinko, Savannah River Technology Center, August 1999.
- Stabilization of Rocky Flats Pu-contaminated ash within chemically bonded phosphate ceramics, Journal of Nuclear Materials, A.S. Wagh et al., vol 265, pp 295-307, 1999.
- Cement and Concrete Research, Disappearance of Oxygen in Concrete Under Irradiation; The Role of Peroxides in Radiolysis, P.Bouniol and A. Aspart, Vol. 28, No. 11, pp. 1669-1681, 1998.
- EPRI NP-6244, Evaluation of Hydrogen Diffusion from Low-Level Radioactive Waste Containers, C.F. Smith and L.B. Phillips, Science Applications International Corp., Pleasanton, California, February 1989.
- LA-UR-99-3053, Materials Identification and Surveillance: June 1999 Characterization Status Report, Los Alamos National Laboratory, Richard Mason et al., June 1999.
- DOE-STD-3013-99, Stabilization, Packaging, and Storage of Plutonium-Bearing Materials, US Department of Energy, Washington, DC, November 1999.
- NUMEC P-80, Progress Report for Development of Plutonium-Bearing Fuel Materials, Nuclear Materials and Equipment Corporation, Apollo, Pennsylvania, September 1961.
- The Plutonium Handbook, Vol II, Gordon and Breach Science Publishers, Inc., O.J. Wick, editor, Plutonium Ceramic Fuel Fabrication by I.D. Thomas, pp. 621 - 641, 1967.
- LA-12999-MS, Plutonium Dioxide Storage: Conditions for Preparation and Handling, J.M.Haschke and T.E. Ricketts, Los Alamos National Laboratory, August 1995.
- Journal of Colloid and Interface Science, Water Vapor Adsorption on Plutonium Dioxide, J.L. Stakebake and L.M. Steward, Vol. 42, Number 2, Feburary 1973.
- ARH-1153, Ceramic Properties of PuO2, O.R.H. Rasmussen, Atlantic Richfield Hanford Co, March 1959.
- RFP-503, Properties of Plutonium Dioxide, J.D. Moseley and R.O. Wing, The Dow Chemical Company, Rocky Flats Division, August 1965.
- Journal of Alloys and Compounds, Adsorption of water on plutonium dioxide, J.M.Haschke and T.E.Ricketts, 252(1977), pp 148-156.
- Journal of Colloid and Interface Science, Water Vapor Adsorption on Plutonium Dioxide, J.L. Stakebake and L.M. Steward, Vol. 42, Number 2, Feburary 1973.
- ARH-1153, Ceramic Properties of PuO2, O.R.H. Rasmussen, Atlantic Richfield Hanford Co, March 1959.
- RFP-503, Properties of Plutonium Dioxide, J.D. Moseley and R.O. Wing, The Dow Chemical Company, Rocky Flats Division, August 1965.
- Journal of Nuclear Materials, The Storage Behavior of Plutonium Metal, Alloys, and Oxide, J.L. Stakebake, vol.38, pp. 241-259, 1971.
- Journal of Nuclear Materials, A review of the oxidation of uranium dioxide at temperatures below 400°C, R.J. McEachern and P.Taylor, vol. 254, pp. 87-121, 1998.
- The Chemistry of Uranium, J.J. Katz and E. Rabinowitch, McGraw-Hill, 1951.
- Personal Communication, Alan Icenhour to Ron Livingston, Moisture Adsorption of UO2, U3O8 and UO3, January 2000.
- A review of the oxidation of uranium dioxide at temperatures below 400°C, R.J. McEachern and P.Taylor, Journal of Nuclear Materials, vol. 254, pp. 87-121, 1998.
- Uranium Dioxide: Properties and Nuclear Applications, Edited by J. Belle, Naval Reactors, US Atomic Energy Commission, 1961.
- LA-13236-MS, White Paper on Possible Inclusion of Mixed Plutonium-Uranium Oxides in DOE-STD-3013-96, J.M. Haschke et al., November 1997.
- SRT-ATS-2000-00028, N.M. Askew to R.R. Livingston, Oxidation Kinetics for Uranium Dioxide, 2/2000.
- Westinghouse Hanford Internal Memo, C.S. Sutter to J.F. Washburn, Technical Evaluation of Receipt of 324 Building Material (with attachments), July 1988.
- Personal Communication, Jeffrey Schaade to Ron Livingston, Re[2]: Completion of DDF-1 OSA…, May 2000.
- AERE-M 1644, Gas Evolution From Solid Plutonium-Bearing Residues During Storage, D.J. Hodkin, R.S. Pitman, and P.G. Mardon, Atomic Energy Research Establishment, 1965.