WSRC-RP-2002-00530
Gibbsite/Bayerite and Uranium in Tank 41H
C. J. Martino and F. F. Fondeur
Westinghouse Savannah River Company
Aiken, SC 29808
Keywords: Tank Farm, Criticality, Salt Dissolution, Low Curie Salt, Aluminum
Summary
A brainstorming session was held on September 10, 2002 to discuss the issue of the aluminum trihydroxide phase (gibbsite and/or bayerite) separation from uranium in Tank 41H inhibited-water-insoluble solids. If a Nuclear Criticality Safety Evaluation (NCSE) for salt dissolution is to credit the hydrogen atoms in aluminum trihydroxide for moderation, an assessment is needed to evaluate if conditions during salt dissolution, and preferably during heel removal, could adversely affect the moderating effects of gibbsite/bayerite with respect to uranium phases. This report outlines the potential mechanisms for the chemical and physical separation of gibbsite from that of uranium in the insoluble solids in Tank 41H. Several of these mechanisms are not applicable to in-tank salt dissolution, while the others can be avoided operationally by limiting hydroxide concentration in the dissolution fluid added to ≤0.016 M and tank temperature to 50°C.
Introduction
Recently, SRTC characterized a Tank 41H salt-well criticality sample to determine the ratio of several neutron poisons to the equivalent 235U after contacting the sample with a large (9:1) mass of 0.016 M NaOH solution. Results revealed that the inhibited-water-insoluble solids was composed primarily (> 79.8 wt. %) of aluminum trihydroxide, identified as chiefly gibbsite or bayerite (Al(OH)3).1 Previous samples taken from saltcake tanks have generally found sufficient quantities of neutron poisons to prevent accumulation of critical masses within the tank upon salt dissolution. For this sample, there was not sufficient neutron poisons, such as Na and Fe, in the remaining insoluble solids to conclude that a critical mass of enriched uranium could not accumulate. An alternate method of ensuring criticality safety allows for the quantity of hydrogen associated with gibbsite and or bayerite (e.g., Al(OH)3).2 This method requires that the atomic ratio of hydrogen to uranium to be >2250:1
The session outlined the inputs and assumptions of the NCSE for Tank 41H dry saltcake. While these are acceptable for the dry saltcake NCSE, they require justification for use in a salt dissolution NCSE. The main concern is whether the gibbsite can be dissolved away, transformed, or otherwise separated from the uranium present in insoluble Tank 41H solids during intentional salt dissolution.
Inputs/Assumptions in the NCSE for Tank 41H Dry Saltcake
The following are the dry salt NCSE assumptions that were identified in the brainstorming meeting:
- The uranium enrichment is 66%.
- Al is present in the inhibited-water-insoluble solids from Tank 41H salt dissolution as gibbsite/bayerite [ Al(OH)3]. This was shown by mass balance. 1
- The hydrogen in gibbsite/bayerite provides a moderator. The atom ratio of hydrogen in gibbsite to 235U is 4339:1 for the insoluble solids resulting from dissolution of the tank 41H sample, which is much greater than the required H:235U atom ratio of >2250:1.
- The insoluble solids from dissolution of Tank 41H saltcake are uniform mixtures of uranium and gibbsite/bayerite.
These assumptions must be justified for use in a salt dissolution NCSE. The first two assumptions are not challenged in this memorandum, and these are applicable to both a dry salt NCSE and a potential Tank 41H salt dissolution NCSE. The third and fourth input/assumption require that the aluminum trihydroxide and the uranium solids are uniform and inseparable. To support third assumption, we identified and addressed several mechanisms that could result in partial dissolution or dehydration of gibbsite without a major impact on the insoluble uranium. To justify the fourth assumption, we addressed the issue of physical separation by settling.
Mechanisms to Remove Gibbsite/Bayerite from Fissile Uranium
The following are the concepts that were identified as potential mechanisms for the in-tank separation of gibbsite/bayerite and uranium:
- Dissolution of gibbsite/bayerite by increased caustic concentration or other change in solution chemistry.
- Conversion (dehydration) of gibbsite/bayerite to boehmite by either heat or radiation.
- Physical separation accomplished by suspension and settling of particles.
In the following sections, these potential mechanisms are explained and addressed. Based on the technical information on hand, recommendations are proposed to aid in avoiding the separation of uranium and gibbsite/bayerite during in-tank salt dissolution.
Aluminum Chemistry in Tank Waste
Dissolved aluminum is usually thought to be primarily present in alkaline waste solutions in the form of soluble aluminate or aluminum hydroxide ion (either AlO2- or Al(OH)4-). In actuality, solutions of this type contain a complex mixture of many different aluminum-containing ions, including oligomers of the aluminate ion as well as complexes with other ions. Depending on the solution composition, the aluminate could be in equilibrium with several solid phases: sodium aluminate salt, amorphous aluminum hydroxide, or several crystalline aluminum hydroxide phases. The common crystalline forms of aluminum hydroxide that are recognized in sludge and saltcake insoluble solids are gibbsite (Al(OH)3, aluminum trihydroxide), bayerite (also aluminum trihydroxide, a polymorph of gibbsite), and boehmite (AlOOH, aluminum oxide hydroxide). There are other aluminum trihydroxide crystal phases (nordstrandidte) and aluminum oxide hydroxide crystal phases (diaspore), but these later forms are not believed to be important stable phases at typical Savannah River Site (SRS) high level waste (HLW) chemistries.
Dissolution of Gibbsite/Bayerite
Based on the analysis of the saltcake sample, there is sufficient insoluble gibbsite and/or bayerite to moderate the enriched uranium during dissolution of saltcake in Tank 41H. This situation could change if in-tank saltcake dissolution is performed at conditions favorable to the dissolution of gibbsite. Gibbsite becomes more soluble as the free hydroxide concentration and/or temperature is increased. Temperature and caustic concentration regimes like those of the processes developed to dissolve the gibbsite and boehmite from sludge before processing in DWPF3,4 should be avoided.
Gibbsite exhibits low solubility in aqueous solutions ranging from pH 4 to 10, reaching a minimum solubility at near neutral pH values (see Figure 1).5 The solubility of gibbsite increases with an increase in the concentration of base (see Figure 2a). From Figure 2a, the solubility of gibbsite is a function of the hydroxide concentration in the dissolution fluid and the temperature.6The presence of other anions, such as NO3-, PO43-, SO42-, CO32-, F-, Cl-, and many organic anions, has been shown to increase the concentration of aluminate ion in the solution phase and thus would promote the dissolution of gibbsite.7 The most abundant component of HLW saltcake is NaNO3, so the influence of NO3- concentration on the solubility of gibbsite (see Figure 2b) is significant.8 Figure 3 displays the gibbsite and sodium aluminate solubility tests that were performed in concentrated solutions of NaNO3, NaNO2, Na2SO4 and Na2CO3 at ratios near those in typical dissolved salt supernates.9 In these saturated salt solutions, gibbsite solubility was seen to be independent of temperature over the range of 20°C to 80°C, but was much greater than the gibbsite solubility in the absence of other salts at 30°C and a comparable free hydroxide concentration. There have been several other gibbsite solubility studies in concentrated NaOH/NaNO3/NaNO2 solutions applicable to dissolved HLW salt.10,11 One such parametric study found that, although fluoride ion is a known complexant of aluminum, there was not a statistically significant effect of the fluoride concentration on gibbsite solubility in concentrated salt supernates typical of tank farm waste.8 Thus, the enhanced gibbsite solubility described by the concentrated salt solution in Figure 3 captures the gibbsite solubility for HLW salt solutions derived from saltcake with other minor anions present.
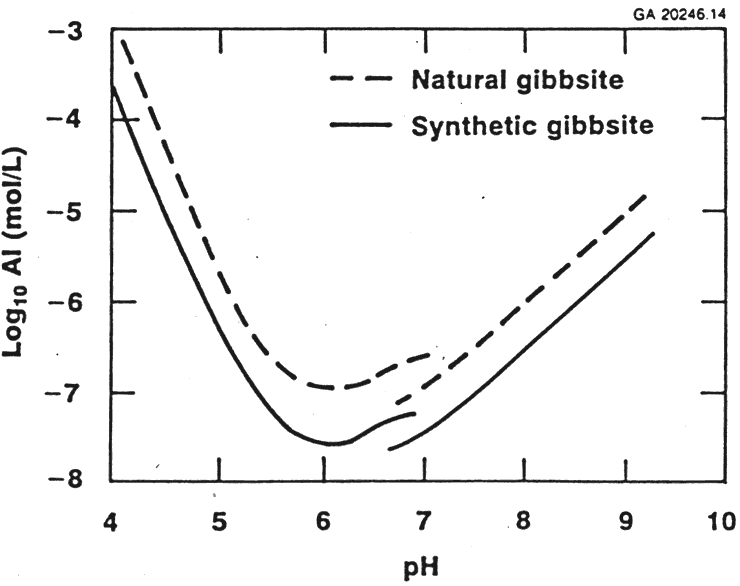
Figure 1: Solubility of Gibbsite at Near-Neutral pH. (Ref. 5)
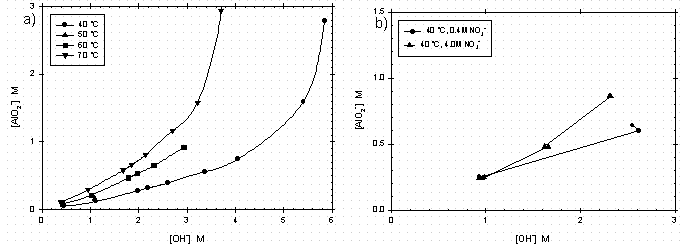
Figure 2: Solubility of Gibbsite in Sodium Hydroxide Mixtures as a
Function of Temperature (Left, Ref. 6) and Solubility of Gibbsite in
Sodium Hydroxide and Sodium Nitrate Mixtures at 40°C
(Right, Ref. 8).
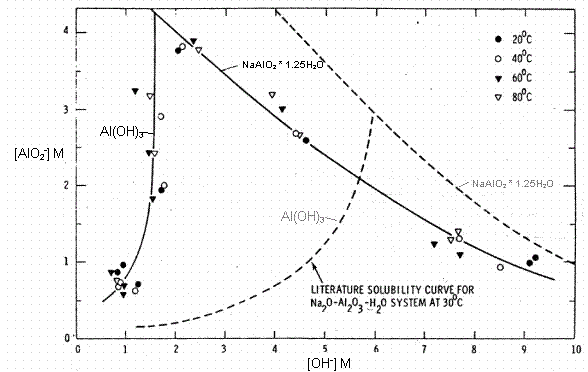
Figure 3: The Solubility of Gibbsite (Left Portion of Curves) and Sodium
Aluminate (Right Portion of Curves) as a Function of Free Hydroxide for
a Saturated Sodium Na-OH-AlO2-NO3-NO2-SO4-CO3
System (Solid Curves, 11M Na+) and the Ternary Na-OH-AlO2
System (Dashed Curves). (Ref. 9)
Figure 3 also provides an indication of conditions that should be avoided to limit gibbsite dissolution during saltcake dissolution. The left portion of the solid curve dictates gibbsite solubility as a function of hydroxide concentration. Unfortunately, the most applicable portion of this curve (<1M OH) is not well developed. In concentrated salt solutions, the solubility of gibbsite is increased greatly as the solution free hydroxide concentration is raised above 1M. Since there is a large mass of aluminum predominantly present in the bulk saltcake as the more soluble salt NaAlO2, gibbsite dissolution will not be favored if the free hydroxide concentration is kept low. The effect of the saturated nitrate, nitrite, sulfate, and carbonate salt solution used to develop Figure 3 increases the gibbsite solubility to such an extent that it would likely mask other impacts of minor components. At later stages of the dissolution and tank cleaning as the inventory of other salts is reduced, the gibbsite solubility would be reduced to a level closer to that represented by the dashed curve in Figure 3. From this figure, it is clear that the dissolution of salt with a high hydroxide dissolution fluid (>1M) could lead to significant gibbsite dissolution.
The quantity of gibbsite relative to uranium in the insoluble solids resulting from the washing of the Tank 41H sample is the basis for the NCSE. The insoluble solids preparation scheme dissolved the bulk of the saltcake through three contacts with inhibited water (0.016 M NaOH). In general, each contact used a 3:1 mass ratio of inhibited water to the original sample weight, for an approximate total mass ratio of 9:1. The in-tank dissolution process will use much less dissolution fluid, thus the sample washing was conservative with respect to gibbsite dissolution. The technology base of the Tank 41H insoluble solids composition is limited because the solids preparation was performed using only a single type of dissolution fluid (0.016 M NaOH). Thus, we can not currently recommend addition of dissolution fluid with a hydroxide concentration greater than 0.016 M. The use of low levels other corrosion inhibitors in the dissolution fluid, such as NO2-, will not affect gibbsite solubility due to the large concentration of other ions present during salt dissolution.
The Tank 41H insoluble solid preparation temperature was nominally 25 to 30°C, but evidence suggests that dissolution at a somewhat higher temperature will not significantly influence amount gibbsite/bayerite in the resulting insoluble solids. Figure 3 demonstrates that gibbsite solubility in concentrated HLW brine is not significantly increased with increasing temperature over the range of 20 to 80°C. Additionally, laboratory-scale salt dissolution experiments performed at both 21°C and 50°C on several Hanford tank samples illustrate that temperature does not significantly affect the amount of aluminum remaining in the resulting solid phase.12 Thus, we conclude that in-tank salt dissolution at temperatures up to 50°C will not significantly reduce the quantity of gibbsite.
Some of the aluminum trihydroxide that was found in the inhibited-water-insoluble solids may have been precipitated upon contact of the saltcake with inhibited water due to the lowering of the solution hydroxide concentration. Because the low-Curie salt process will dissolve roughly the same starting saltcake material by the addition of a fluid with a [OH-] ≤ 0.016 M, any such gibbsite precipitation would also occur during the in-tank salt dissolution process. Although gibbsite may be both dissolved and precipitated during the dissolution process, the aluminum trihydroxide observed in the insoluble solids during sample characterization will be present in the resulting insoluble solids from in-tank dissolution.
Based on analysis of the Tank 41H salt well sample1 and reported gibbsite solubility studies, an adequate amount of gibbsite will be present in the solids resultant of salt dissolution provided the temperature is maintained below 50°C and the hydroxide concentration of the dissolution fluid added is maintained at or below 0.016 M.
Conversion of Gibbsite/Bayerite to Boehmite
The solubility of boehmite is less than gibbsite at normal tank farm conditions, which is beneficial to hinder dissolution as outlined in the previous section. However, the transformation of gibbsite into boehmite is not desired because such a conversion results in the dehydration of the crystalline phase, which lowers the atomic ratio of H:235U. We identified two potential paths for the in-tank conversion of gibbsite to boehmite.
The first conversion mechanism is the heating of the gibbsite in saltcake to a temperature at which the transformation to boehmite is favorable. Figure 4 shows that boehmite does not become the favored aluminum phase until the temperature is above about 80°C.5 The dissolution of tank farm saltcake is slightly endothermic, so the thermal conversion of gibbsite into boehmite (ie. 80°C) is not expected in the absence of heating.
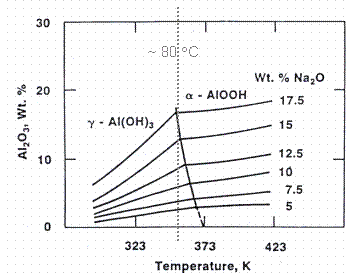
Figure 4: Phase Diagram for Gibbsite (g
-Al(OH)3) and
Boehmite (a -AlOOH) at Caustic Conditions.
(Ref. 5)
The second mechanism identified is the radiation-driven conversion of gibbsite to boehmite. This is a very slow process that occurs at the surface of dry gibbsite particles. This radiolytic gibbsite conversion mechanism does not apply to salt dissolution because the gibbsite would have to be left completely dry for long periods of time before the gibbsite particle surfaces will be significantly dehydrated. Unpublished results indicate that the amount of gibbsite present in sludges that are surrounded by supernate actually increases with irradiation.13 Due to chemistry changes within the interstitial fluids in the sludge, soluble aluminate residing in the interstitial fluid is radiolized to gibbsite at a faster rate than gibbsite undergoes radiolysis to boehmite.
Thus, significant conversion of gibbsite/bayerite to boehmite will be avoided by keeping the temperature below 80°C.
Physical Separation by Settling
An important assumption in the NCSE is that the uranium and gibbsite/bayerite would be dispersed uniformly throughout the inhibited-water-insoluble solids. In the neutralization of acidic canyon streams to form sludge, there is little evidence that gibbsite and uranium coprecipitate. The precipitation behavior of gibbsite and uranium solids from the cooling of supersaturated HLW evaporator condensate is not known (potentially, they are more associated than in sludge). For conservatism, the gibbsite and the uranium are assumed to be dispersed in separate particles. If the uranium and gibbsite/bayerite particles do not settle at comparable rates, stratified layers could be formed if the dissolution process allows for sedimentation (e.g., sweeping solids into the pump well and allowing them to settle below the pump, or mixing and settling a heel of insoluble material at the completion of salt dissolution).
During the Tank 41H insoluble solids preparation from the recent (June 2002) sample, visual observations were inconclusive as to the uniformity of the uranium dispersion.1 The centrifuged insoluble solids did appear to have a thin layer near the surface of the centrifuged solids that was slightly darker than the bulk material. Pure gibbsite is a white powder, and the light gray appearance of the bulk material likely consisted of gibbsite with various impurities. Sodium diuranate solids are yellow in color. The slightly darker layer was not analyzed separately from the bulk insoluble material. Uranium comprised such a small portion of the inhibited-water-insoluble solids (< 0.2 wt.%) that precluded visual observation of a uranium rich layer in the settled solids.
For an earlier (1994) Tank 41H surface sample, Scanning Electron Microscopy with X-ray Energy Dispersive Spectroscopy (SEM/EDS) was performed on the insoluble solids.15 This analysis gives a picture of the particles (size and morphology) and a relative elemental composition of the individual particles. The particle sizes determined for the insoluble solids averaged 3 mm, with the particle size range of 0.9 mm to 9.5 mm. Using these particle sizes and knowledge of the particle densities, the settling rates were estimated using Stokesí Law. The Stokesí Law particle settling rate is directly proportional to the difference in particle and fluid density and to the square of the particle radius (assumes spherical particles). The relatively wide distribution of bulk particle sizes will not allow for the separation of uranium from the bulk material by gravity settling. The particle sizes and settling rates of the uranium particles in the surface sample can be directly extended to the salt-well sample since they were formed by the same processes of waste evaporation, concentration, and cooling. The bulk particles in the surface sample, however, were not shown to be gibbsite or bayerite.
A previous study of uranium precipitation from evaporated alkaline HLW simulant yielded sodium diuranate particles with a particle density of 3.93 g/cm3. Gibbsite has a density of 2.42 g/cm3 and bayerite has a density of 2.53 g/cm3.5 SEM or other particle size analysis was not performed on the Tank 41H salt-well criticality sample. We do not have other specific knowledge of gibbsite particle size precipitated from evaporation of our HLW solutions, and the gibbsite/bayerite particle sizes are likely somewhat a function of the conditions at which they are formed. Using knowledge of the Bayer process, gibbsite particles are typically formed from caustic salt solutions with particle sizes of 1 to 10 mm, with larger agglomerates possible (hundreds of microns).16,17 Factoring in agglomerates, the gibbsite particles would likely have a large enough range of settling rates to easily bound the uranium settling rate and thus be inseparable form the uranium. Additionally, the typical gibbsite precipitated in Bayer liquors has a particle size range that is similar to that of the bulk particles in the Tank 41H surface sample.
Based on extension of the particle size characterization and settling velocity calculations previously performed on a Tank 41H surface sample, the uranium would not be segregated from the bulk insoluble solids by gravity settling.
Conclusions
Controlling the operating temperature and added hydroxide concentration should allow for both the prevention of transforming gibbsite to boehmite and the suppression of gibbsite dissolution. We believe that there is sufficient overlap in the particle size distribution (and thus the settling rates) of gibbsite and uranium particles that gravity settling will not be an effective separation mechanism. The following summarizes the recommendations of this analysis:
- Based on the Tank 41H sample results,1 no significant decrease in the amount of gibbsite in the insoluble solids resulting from salt dissolution is expected as long as the dissolution fluid has [OH-] ≤ 0.016 M.
- No significant decrease in the amount of gibbsite in the insoluble solids resulting from salt dissolution is expected for process temperatures up to 50°C.
- No significant conversion of gibbsite into boehmite is expected at temperatures below 80°C.
- Previous Tank 41H sample analysis combined with Bayer process knowledge of typical gibbsite precipitation suggest that gibbsite and the uranium will not segregate.
Acknowledgements
In addition to the authors, the following are the contributors to the September 10 brainstorming meeting: Scott Rosencrance, Bill Wilmarth, Bill Van Pelt, and Walt Tamosaitis of WPTS, Joe Carter and Fatina Washburn or HLW, and Kristan McCoid of WSMS. We thank Jonas Addai-Mensah of the University of South Australia for his helpful conversations about the chemistry of aluminum hydroxides. We also thank David Hobbs for his discussions concerning his previous work with uranium and the earlier Tank 41H sample..
References
- C. J. Martino, "Tank 41H Salt-Well Sample Analysis," WSRC-TR-2002-00343,
Revision 0, August 15, 2002.
- K. J. McCoid, "Nuclear Criticality Safety Evaluation: Tank 41
Dry Saltcake," N-NCS-H-00147, Revision 1, September 6, 2002.
- E. J. Weber, "Aluminum Hydroxide Dissolution in Synthetic Sludges,"
DP-1617, March 1982.
- J. T. Granaghan, "Technical Data Summary: In-Tank Sludge Processing,"
DPSTD-84-100, April 24, 1984.
- K. Wefers and C Misra, "Oxides and Hydroxides of Aluminum,"
Alcoa Technical Paper No. 19, Revised, Alcoa Laboratories, Pittsburgh,
PA, 1987.
- A. S. Russel, J. D. Edwards, and Cyril S. Taylor, "Solubility
and Density of Hydrated Aluminas in NaOH Solutions," Journal of
Metals, October 1955.
- P. H. Hsu, "Aluminum Hydroxides and Oxyhydroxides", Minerals
in Soil Environments, J. B. Dixon, S. B. Weed, and R. C. Dinauer, eds.,
(Soil Science Society of America: Madison, WI), 1989.
- D. L. Herting, T. L. Welsh, and D. A. Reynolds, "Gibbsite Equilibrium
in Simulated Liquid Waste," SD-WM-TI-211, 1985.
- G. S. Barney, "Vapor-Liquid-Solid Phase Equilibria of Radioactive
Sodium Salt Wastes at Hanford," ARH-ST-133, January 1976.
- A. R. Felmy, J. R. Rustad, M. J. Mason, and R. de la Bretonne, "A
Chemical Model for the Major Electrolyte Components of the Hanford Waste
Tanks: The Binary Electrolytes in the System: Na-NO3-NO2-SO4-CO3-F-PO4-OH-Al(OH)4-H2O,"
PNL,SA-23952, August 1994.
- S. M. Sterner, A. R. Felmy, P. K. Melethil, M. J. Mason, and J. R.
Rustad, "Extension of the ESP Model to High Base and High Aluminate
Concentration," PNNL report prepared for Westinghouse Hanford Company,
May 31, 1996.
- D. L. Herting, "Saltcake Dissolution FY 2001 Status Report,"
HNF-8849, Revision 0, September 2001.
- F. F. Fondeur, D. T. Hobbs, and S. D. Fink, "The Effect of Sludge
Aging on the Aluminum Speciation of Sludge," WSRC-TR-2002-00465,
(Draft).
- D. T. Hobbs, "Precipitation of Uranium and Plutonium from Alkaline
Salt Solutions," Nuclear Technology, 128, 103-112, 1999.
- D. T. Hobbs, "Characterization of the Tank 41H Saltcake Insoluble
Solids (U)," WSRC-RP-94-1213, Revision 0, October 31, 1994.
- J. Addai-Mensah, "Surface and Structural Characteristics of Gibbsite
Precipitated from Pure, Synthetic Bayer Liquor," Min. Eng., 10,
81-96, 1997.
- C. Sweegers, H. C. deConinck, H. Meekes, W. J. P. van Enckevort, I. D. K. Hiralal, and A. Rijkeboer, "Morphology, Evolution and Other Characteristics of Gibbsite Crystals Grown From Pure and Impure Aqueous Sodium Aluminate Solutions," J. Crystal Growth, 233, 567-582, (2001).