WSRC-MS-99-00501
Separation Membrane Development
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Abstract
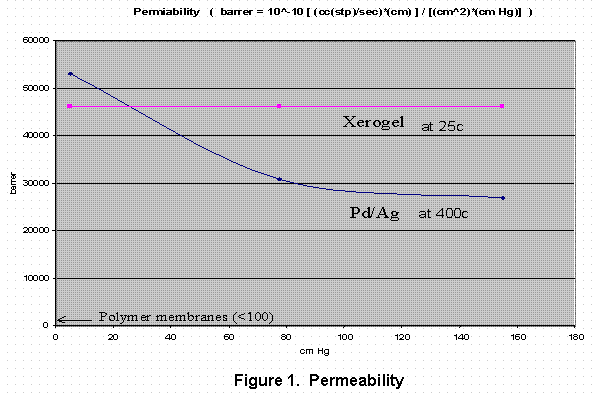
A ceramic membrane has been developed to separate hydrogen from other gases. The method used is a sol-gel process. A thin layer of dense ceramic material is coated on a coarse ceramic filter substrate. The pore size distribution in the thin layer is controlled by a densification of the coating materials by heat treatment. The membrane has been tested by permeation measurement of the hydrogen and other gases. Selectivity, 10,000 to 50,000, of the membrane has been achieved to separate hydrogen from carbon monoxide. The permeability constant of hydrogen through the ceramic membrane was about 46,000 Barrer at the room temperature, which is about the same as Pd-Ag membrane at 400c.
Background
Billions of standard cubic feet of hydrogen are consumed every day by the refinery industry alone. Demand for hydrogen has continued to grow in recent years. In hydrogen recovery and production, separation of hydrogen from other gases is an important part of the process. Pressure swing adsorption (PSA) is the main separation method but is an energy intense operation. Metal alloys or composite metal membrane have been used for hydrogen purification, but metal is sensitive to poisonous gases. A ceramic membrane, inert to poisonous gas, is desirable.
The sol-gel encapsulated metal hydrides, developed at the Savannah River Technology Center have solved the problem of decrepitation of metal hydride particle. The pore size of the sol-gel coating can be tailored to discriminate between H2 (2.89A) and CO (3.76A) on the basis of molecular size in much the same manner that silica membranes have been shown to have the ability to separate hydrogen and nitrogen.
Overall Approach
The overall objective is to develop and demonstrate a thin dense glass coating on a coarse ceramic substrate which allows hydrogen to easily permeate through, but no other gases. The glass coating on coarse substrate is by the sol-gel process. The coarse substrate can be metal frit or ceramic filter materials. The pore size of the thin dense layer is controlled by the conditions of the sol-gel coating process and the heat treatments. The heat treatment including microwave makes the coating dense.
A parallel approach has been taken:A composite metal hydride has been produced. Tests show that the selectivity of hydrogen to carbon monoxide is in the rage of 10,000 to 50,000. It can be used for an absorption based hydrogen separation process.
The current focus is on the development of a ceramic membrane filter.
Filter Fabrications
A. Sol-Gel Formulations
The sol-gel technique has been well published in the literature (1). The technique starts with the hydrolysis of an organo-metallic compound. The hydrolyzed compound is polymerized via water and alcohol condensations and dried by removing water and the solvent. An acid or base is used to catalyze the polymerization reaction. The heat treatment following the drying step is used to further modify the final product. The reactions involved are generally as follows:
Organo-metallic Compound solution in alcohol, M-(OR)4 in ROH, and water-alcohol solution are mixed to make several reactions:
Hydrolysis reaction: (OR)3-M-OR + HOH = (OR)3-M-OH + ROH
Water Condensation: (OR)3-M-OH + (OR)3-M-OH = (OR)3-M-O-M-(OR)3 + H2O
Alcohol Condensation:(OR)3-M-OH + (OR)3-M-OR = (OR)3-M-O-M-(OR)3 + ROH
where M is metal such as Si, Al, or Ti and R for an alkyl group, (-CxH2x+1) in most case -C2H5 or -CH3.
The first solution is formed by mixing one part ethanol into two parts tetraethyl orthosilicate (TEOS). The second solution is formed by mixing 2~5 parts of ethanol to one part of water. Acidity of the second solution is adjusted by adding HCl until the PH is in the range of 1 to 2.5. The second solution is added to the first solution while stirring continuously to form sol. The sol is then covered and allowed to age for 2~24 hours which allows the sol, initially a water-like consistency, to become viscous. This viscous sol is used to make a thin coating.
B. Sol-Gel Filter Formation
The viscous sol is drip coated on a course substrate (silica or alumina). The next step is drying to evaporate water and alcohol formed by a polymerization. The filter is then allowed to gel completely for a few days. The drying can be done either by evaporation at room temperature or in a ventilated oven at elevated temperatures. In general, elevated temperatures give broader pore distribution.
C. Heat Treatment
Heat treatments create densification of the sol-gel coating. The dried sample can be heat treated under vacuum to vary the pore size. While the sample is under the vacuum, its temperature is raised to a target value and maintained for 2 hours. The target temperatures used are from 200 to 600C. Further treatments are done by a microwave oven.
Experimental Results
A. Pore Distribution
Pore size distributions are measured on sol-gel coating materials by BET gas adsorption. This method can not be used to measure pore size smaller than 15 A. True selectivity depends on the size of the narrow channels between the pore to pore, not on the pore size itself. However, the distribution of pore size may indicate the quality of the coating. Typical pore size distributions are summarized in Table 1. Coating with a narrow pore size distribution has good selectivity over 10,000, but the coating with broader distribution shows poor selectivity of less than 5.
Table 1. Pore Size Distribution (in nm)
|
High Selectivity Coating |
Low Selectivity Coating
|
||
|
Peak Location
|
Half Width
|
Peak Location
|
Half Width
|
|
2.5
|
1.2
|
1.3
|
4.5
|
|
1.3
|
1.2
|
2.3
|
30.
|
|
1.7
|
0.5
|
3.2
|
30.
|
|
1.9
|
1.0
|
8.0
|
60.
|
|
2.2
|
0.8
|
|
|
B. Performance Testing
The permeation rates of hydrogen through the filter are measured to test the performance of the sample. The pressurized hydrogen gas is allowed to flow through the filter to a constant lower pressure. The flow rates are then determined. The results are compared with the flow rate of a typical Pd-Ag membrane in Figure 1. At the low pressure, samples tested have about same permeability constant as the Pd-Ag membrane.

Conclusion
The ceramic membrane filter can be produced by a sol-gel process. Tests show promising results. Improvement on a defect free, large filter is in progress.
References