This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available to DOE and DOE Contractors from the Office of Scientific and Technical Information, P. O. Box 62 Oak Ridge, TN 37831; prices available from (423) 576-8401.
Available to the public from the National Technical Information
Service, U.S. Department of Commerce, 5285 Port Royal Road, Springfield, VA
22161.
Methanotrophic bacterial populations were quantified in an aquifer that was amended with air (oxygen), methane, triethyl-phosphate, and nitrous oxide to evaluate their effectiveness to stimulate aerobic bioremediation of vinyl chloride (VC), dichloroethylene, and trichloroethylene (TCE). Contaminants in groundwater resulted from leachate originating from a nearby landfill. Groundwater samples were taken during gas injection and analyzed for changes in bacterial populations. The methanotrophic populations were monitored in groundwater using direct fluorescent antibodies (DFA) and the most probable number (MPN) technique. Acridine orange direct counts (AODC) were used to determine the total bacterial population. Methanotrophic populations increased significantly in groundwater during the course of gaseous nutrient injections. As methanotrophic bacteria reached a maximum population in 3-4 days, contaminant levels (TCE) decreased. Cis-dichloroethylene (c-DCE) demonstrated a transient increase in concentration during the experiment but decreased rapidly over the course of the experiment. The total number of groundwater microorganisms did not change, indicating a selective stimulation of the methanotrophic bacterial population. These bacterial data were compared to physical parameters (pH, dissolved oxygen, redox) and contaminant (TCE , c-DCE, VC) concentrations within the saturated and unsaturated zone to reveal the efficiency of the system. The loss of contaminants appears to be due to cometabolic biodegradation through biostimulation since loss by volatilization was accounted for and was minimal. This work clearly demonstrates that one can effectively change the subsurface bacterial population in a relatively short period of time.
This project was designed to evaluate the subsurface bacteria as influenced by an in situ bioremediation system. This system was designed for field testing of in situ treatment of groundwater contaminated with chlorinated solvents at the Department of Energy (DOE), Savannah River Site Sanitary Landfill (SLF) (WSRC, 1996). For over 20 years the SLF received paints, thinners, rags soaked with organic solvents including trichloroethylene (TCE) and perchloroethylene (PCE), sanitary waste, construction material, and disposable batteries. Leachates containing TCE were detected in groundwater samples during an evaluation from 1984 through 1993 (SRS, 1996).
Subsurface gaseous nutrient injection has been found to be an effective in situ bioremediation treatment for TCE-contaminated groundwater (Pfiffner et al., 1997). The biodegradation of TCE was found to be sensitive to factors controlling microbial growth including injected methane and nitrogen (Travis and Rosenberg, 1997). In situ biodegradation is a highly attractive technology for remediation because contaminants are destroyed in place, not simply moved to another location or immobilized. Groundwater monitoring of bacteria and contaminant concentrations provides information on the efficiency of this technology. Application of these methane/air mixtures have been demonstrated to stimulate TCE-degrading methanotrophic bacteria in the subsurface (Pfiffner et al., 1997). Results from an investigation to characterize biodegradation and volatilization of TCE in a bioremediation test at the SLF are presented.
The objective of this test was to demonstrate that selective biostimulation can result in preferential changes to the status of subsurface microbial communities. We monitored how groundwater bacteria responded to multiple gaseous nutrient injections to determine the effectiveness of bioremediation of TCE and its byproducts in the SLF.
The SRS is owned by the U.S. DOE and operated by WSRC. The SLF is located within the 310 square mile SRS facility located along the Savannah River, principally in Aiken and Barnwell Counties, South Carolina. The SLF began operating in 1974 and reached capacity in 1987 at which time expansions were added to increase it's capacity to 70 acres.
A pilot-scale gaseous injection remediation test system was designed and installed within the TCE plume to validate the use of in situ biodegradation of chlorinated solvents (WSRC, 1996). The test system consists of three sparge wells, a gas extraction well, and 14 nested monitoring points. Each nested monitoring location within the TCE plume has two mini-wells in the unsaturated soils (one shallow = 10 feet below ground surface [bgs]; one deep = 16 feet bgs) and two mini-wells in the saturated soils (one shallow = 30-40 feet bgs; one deep = 45-55 feet bgs). During this test, air, methane, nitrogen, phosphorous, and helium were injected for approximately 8 hours a day, followed by 16 hours of no injection (for six consecutive days). This injection contained a mixture of 15 SCFM (Standard Cubic Foot per Minute) air blended with 4% methane (CH4), 0.07 % Nitrous Oxide (N2O), 0.007 % to 0.01 % Phosphate (Triethyl Phosphate, TEP (C2H5)3PO) and 1.0% Helium (He) injected as a tracer.
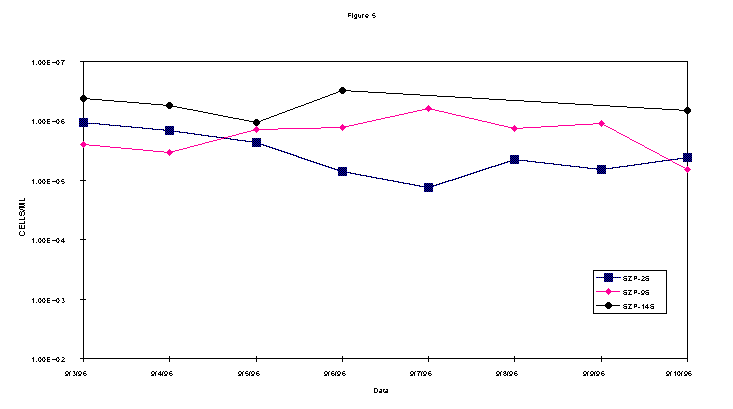
Once gas injection had begun water samples were collected every 24 hours from wells 2s, 9s, and 14s for microbial and groundwater contaminant data. Total bacterial counts were accomplished by the Acridine Orange Direct Count Method (AODC) methodology. Methanotrophic bacteria were quantified using the Direct Fluorescent Antibody (DFA) procedure and the Most-Probable-Number (MPN) technique (Brigmon et al., 1998b). Groundwater samples were analyzed for volatile organic compounds (VOCs) as previously described (Brigmon et al., 1998a). Vadose zone gas samples were taken to monitor possible volatilization.
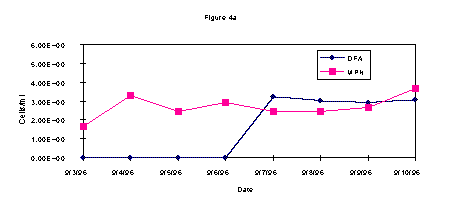
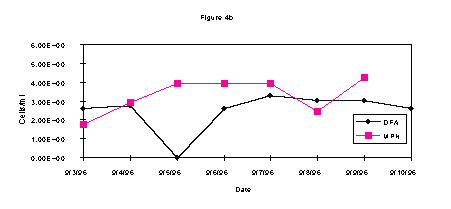
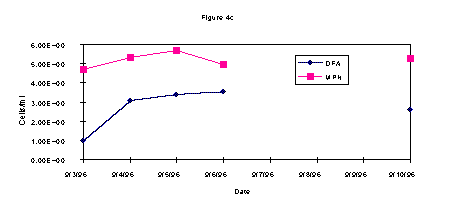
The DFA and MPN results on enumeration of methanotrophic bacteria from wells 2S, 9S, and 14 S are shown in Figures 1a-c. Overall, the data from each well show an increase in the methanotrophic bacteria population on or about midweek. These data are in agreement regardless of the technique employed, i.e. DFA or MPN.



During the test, AODC densities did not significantly change (Figure 2). This lack of increase in the overall bacterial count when compared with the methanotrophic count indicates selective biostimulation during the test. Microbiological data demonstrates that the indigenous methanotrophic population requires three days to adapt to the injection of gaseous nutrients to the subsurface. In comparing the contaminant data with the bacterial densities, it is evident that as the methanotrophic bacteria population increased, the contaminant level decreased. These results show it is likely that the chlorinated hydrocarbons, TCE and c-DCE, were cometabolized by the enriched indigenous methanotroph population.
The analytical results of groundwater samples showed a decrease in the contaminant concentrations over the week of (Figures 3a, b, and c). In all three wells there was an initial increase in the quantity of c-DCE, a byproduct of the microbial biodegradation of TCE. After the middle of the week, this concentration decreased in all wells. The greatest contaminant fluctuation in all three wells was the c-DCE concentrations. Both wells 2S and 9S demonstrated an initial increase of approximately 250 ppb c-DCE that decreased below baseline levels by the end of the testing (Fig 3a and 3b). Well 14s was further from the direct influence of the injection system and results showed a lesser impact on contaminant concentrations including a 40 ppb increase in c-DCE that also decreased by the end of testing (Fig 3c).
Daughter products of TCE biodegradation including 1,2-dichloroethylene (DCE) and vinyl chloride (VC) are present in the SLF groundwater even though these compounds were never disposed of at this site. The presence of these compounds is evidence of natural attenuation of TCE (Brigmon et al., 1998a).
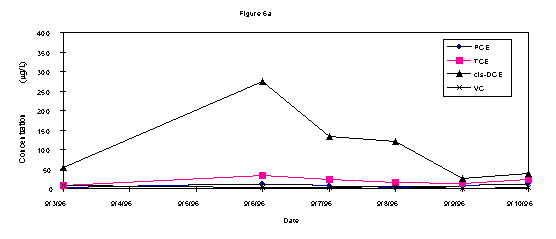
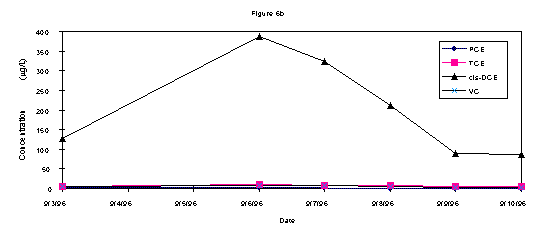
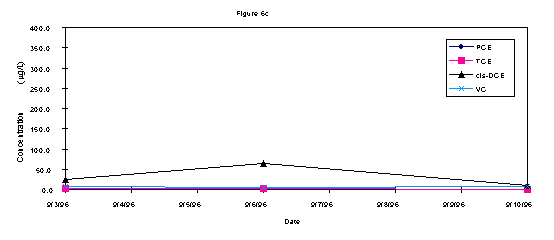
Bouwer (1994) has reported if the amount of c-DCE is greater than 80% of the total DCE, which it is in this case, it is a biodegradation product of TCE. Since monitoring well information (contaminant concentrations at each depth interval for a nested well) did not indicate a contaminant mass transfer from the groundwater to the vadose zone, it is likely that c-DCE was actively biodegraded. The fact c-DCE is present in the groundwater and then after nutrient injection there is a transient increase of c-DCE is further evidence of active TCE biodgradation. In this case there is still TCE and other materials potentially leaching from buried material in the SLF (WSRC, 1996).
Selection of the appropriate technology can be critical to the success of bioremediation applications. A better understanding of the potential mechanisms involved for enhanced microbial degradation in the subsurface and the interaction between groundwater, microorganisms, and contaminants may be useful in extending the application of in situ bioremediation to additional contaminated sites by helping in the technology selection process. This demonstration and report represent another approach in the development of in situ bioremediation technologies.