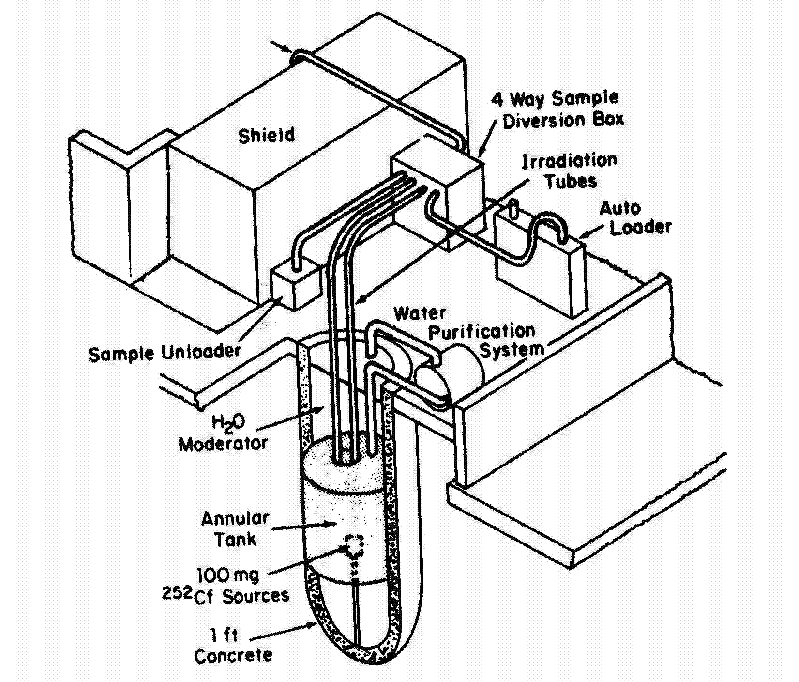
Figure 1. 252Cf Neutron Activation Analysis Facility
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available to DOE and DOE Contractors from the Office of Scientific and Technical Information, P. O. Box 62 Oak Ridge, TN 37831; prices available from (423) 576-8401.
Available to the public from the National Technical Information Service, U.S. Department of Commerce, 5285 Port Royal Road, Springfield, VA 22161.
Neutron activation analysis using low-flux isotopic neutron sources is put to use in addressing areas of applied interest in managing the Savannah River Site. Some of the applications are unique due the site’s operating history and its chemical processing facilities. Because sensitivity needs for many of the applications are not severe, they can be accomplished using a ~6 mg 252Cf neutron activation analysis facility. Overviews of the facility and several example applications are presented.
The Savannah River Technology Center (SRTC) has used a 252Cf neutron-activation-analysis (NAA) facility1 routinely since 1976. Methods for a much larger and more highly automated activation analysis system at the Savannah River Site (SRS) C-Reactor were developed at the 252Cf facility to support the national uranium resource evaluation (NURE) program. In its heyday in the late 1970s and early 1980s, the reactor facility provided fully-automated, resource-driven activation, counting, sample storage and retrieval for over 70,000 soil and sediment samples per year. Uranium was determined by delayed neutron counting, and a broad suite of other activated elements were measured by gamma-ray spectrometry.
Although the C-Reactor facility is no longer in use, the SRTC 252Cf facility continues to provide multi-element analyses on solid and liquid samples. The isotopic neutron source supports applied research programs at SRTC as well as other SRS programs for environmental and waste management customers. Applications have included elemental analysis for samples consisting of metal alloys, sediments, rocks, site process solutions, and many other materials. In addition to direct bulk-sample NAA, numerous radiochemical analyses rely on the facility for production of short-lived tracers, yielding by activation of carriers and small-scale isotope production for separation methods testing.
The present SRTC NAA facility (Fig. 1) holds eight doubly encapsulated 252Cf pods in a zircalloy source holder near the bottom of a 4-meter deep, 1.2-meter diameter tank. The tank contains deionized light water, and the source holder is in the annulus of a second tank filled with D2O. The heavy water tank has a total of eighteen irradiation ports that are arranged in two rings (~5.8 and ~11.2 cm radii) concentric to the source holder. Three of the positions can be pneumatically fed; samples are introduced manually at the remaining fifteen locations. All irradiation positions were designed to accept 13-mL high-density-polyethylene containers ("rabbits"). At one time, the facility contained eight sources having a total 150-mg mass of 252Cf. Its present 252Cf content of about 6 milligrams gives a thermal-neutron flux of about 6x107 n/(cm2 sec) at the highest flux irradiation positions. The heavy water tank minimizes flux gradients across samples and it gives a flux that is only a factor of ~three lower at the outer irradiation ring. Although the flux is low relative to reactor facilities, it continues to offer sufficiently sensitive analyses for many on-site applied research efforts and other SRS programs. Over 40 elements can be detected at low- and sub-part-per-million levels.
Germanium detectors for gamma-ray spectrometry and 3He-filled proportional counters for delayed neutron counting are located adjacent to the NAA tank in a counting vault with a 1.2-meter wall thickness. A pneumatic transfer system allows analysis of elements having short-lived activation products, and automated cyclic irradiations can be performed to enhance sensitivity for short-lived activation and fission products. Samples containing small quantities of fissile materials may be analyzed using this cyclic capability via delayed neutron counting.
Over the years, gamma-ray spectrometry at the facility passed through several generations of computers, software, multichannel analyzers, and germanium detectors. Available germanium detectors presently include three conventional coaxial detectors, a well detector, a small-area- and a large-area-low-energy-photon spectrometer. Conventional nuclear instrument module (NIM) amplifiers and analog to digital converters (ADC) comprise most of the data acquisition hardware, but a digital signal processing (DSP) system is brought to bear for high-count-rate applications. All of the data acquisition systems take advantage of pulse-pile-up rejection and dead-time corrections that are available using present-day commercially available electronics. The DSP electronics offer a clear count-rate-throughput advantage relative to conventional amplifiers and ADCs when count rates are high. For activation products having suitable half-lives, an automated sample changer is also available for more cost-effective counting.
Data acquisition devices are interfaced to Intel-based personal computers through Canberra Industries (CI) acquisition interface modules. CI’s Genie2000 software controls data acquisition, analyzes gamma-ray spectra and simplifies quality assurance. Originally, SRTC applied absolute methods to interpreting counting data; these relied on knowledge of detector efficiencies, neutron energy spectra, neutron cross-sections, etc. to determine elemental contents. Most NAA applications now rely on direct comparisons of results on "unknowns" to those determined on standards.
Other multielement analysis methods, particularly inductively coupled plasma atomic emission (ICP-AE) and inductively coupled plasma mass spectrometry (ICP-MS), now provide impressive sensitivities on dissolved samples that are often better than those available by NAA using low-flux isotopic sources. Despite the low neutron flux, the NAA facility’s sensitivity is adequate for many analytical projects, and it is a more cost-effective choice in many cases because it allows analysis of bulk samples without chemical digestion. For example, materials that may come in contact with stainless steels are frequently tested to determine whether chlorine concentrations are below limiting values set by materials corrosion considerations.
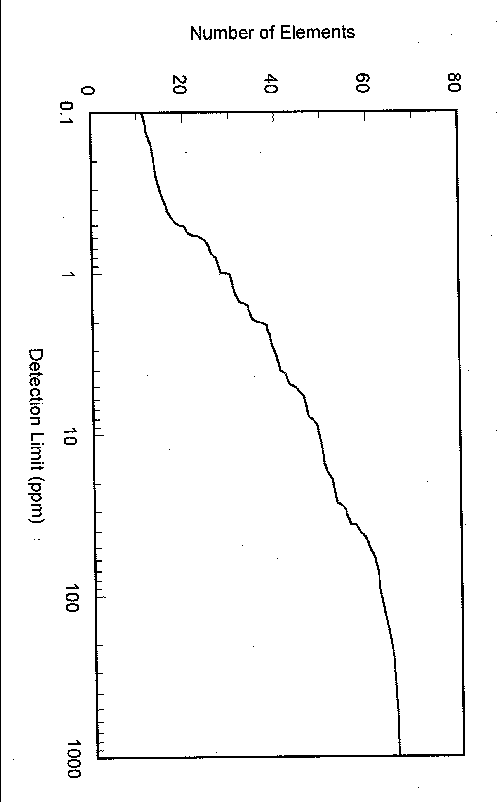
NAA sensitivity is dependent on neutron flux, irradiation time, sample mass, detector counting efficiency and background, and count time. Figure 2 shows sensitivities2 for NAA at the SRTC NAA facility using reasonable activation and counting protocols; the figure assumes that activation and counting times individually are the lesser of two half lives or two days. Most routine analyses, however, receive relatively short exposures and counts while meeting analytical goals. Since counting backgrounds for NAA are matrix dependent due to activation of other species in the matrix, the sensitivities shown are for bounding-case matrices that offer few if any interferences.
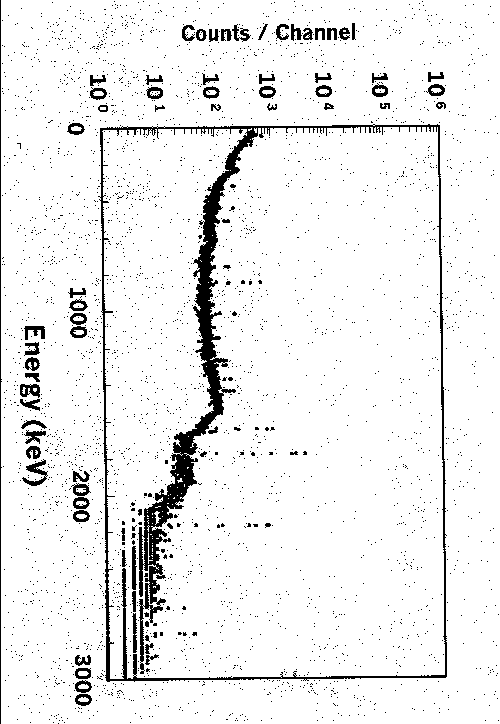
Chlorine analyses have been a mainstay for the facility. These data are used to assure that chlorine concentrations in materials that may come in contact with stainless steels meet technical specifications. On solutions, ion-selective electrode methods are more sensitive for chlorine than SRTC’s NAA facility. However, in solid matrices, NAA becomes competitive with wet chemistry methods when the requirements for digestion require large dilution factors. The NAA facility as it currently stands can easily meet the Savannah River Site’s total chlorine concentration limit of 250 ppm on such matrices as gasket materials, labels, certain metals and alloys, etc. Under optimum conditions, the facility’s chlorine sensitivity is about 18 ppm for a 10-gram sample. However, in a more typical 30-minute irradiation, 5-minute decay period and 16.6-minute count (Fig. 3), the chlorine sensitivity is about 80 ppm. If appreciable levels of elements (such as sodium) were present, they would degrade sensitivity by significantly raising the Compton background below 38Cl’s 1643 and 2168 keV gamma-ray peaks.
Although in less demand than chlorine analyses, iodine and bromine analyses can be carried out similarly. Sensitivities superior to analytical techniques such as ion chromatography or ion-selective electrode are provided for these halides on solution samples of solids that are not easily digested.
Radiochemical separations are required for analysis of beta emitters that have no significant gamma-ray emission intensity. The separations generally require yielding by independent analytical methods to account for losses of analyte in separation processes. Yields may be determined by comparing the amount of carrier or radioactive tracer that is recovered after separation to the known amount added to the sample before commencing the separation. Specific examples of using neutron activation for tracer preparation and for carrier measurements are given in discussions that follow. Because the SRTC NAA facility is a part of a larger analytical organization, numerous analytical methods often are available to measure carriers. Decisions on analytical paths are guided by analysis costs; when it makes economic sense, non-nuclear analytical methods are applied for yielding.
Once preirradiation activity measurements are completed, numerous other radiochemical separations are yielded by neutron activation analysis of elemental carriers. These include analyses for 90Sr, 129I and column-separated rare earth elements.
Analyses for 90Sr, a pure beta emitting fission product having a 30-yr half life, are required to support development of high-level waste vitrification methods, and other samples of interest to waste management and environmental restoration customers. In early efforts, 85Sr tracer measurement data provided yields for 90Sr analyses; however, the presence of 85Sr interferes with beta activity measurements of 90Sr/90Y by liquid scintillation (LSC) and Cerenkov counting. This interference and its resulting sensitivity degradation are avoided by using strontium carrier for yielding. After LSC counting is completed, the same small 7-mL vials used for LSC counting are sealed in NAA rabbits, activated and counted. In this way, yielding is conveniently and cost effectively accomplished by NAA without returning the sample to a radiologically posted laboratory, without reopening the sample and with minimal additional preparation. The SRTC radiochemistry laboratory uses Eichrom Industries extraction chromatography columns for strontium separations. Using 5-mg strontium carrier, an amount recommended by Eichrom to avoid overloading the column, NAA provides accurate yields by measuring the 388 keV gamma rays of the 87mSr product.
129I is a fission product with a half-life of about 16 million years. It is very mobile in the environment due to its low retention in sub-surface soil. 129I activities in environmental samples generally are too low to allow measurement by direct counting methods. In an earlier era when high-flux, in-core (~2x1014 neutrons/(cm2*sec)) reactor irradiations were available at the SRS, 129I in environmental samples was analyzed3 in a sequential scheme that included preconcentration, neutron activation, post-irradiation chemistry and counting of the shorter-lived 130I activation product. The neutron flux at the SRTC NAA facility is too low to determine 129I at typical environmental levels by activation to 130I; however, the facility supports other 129I analyses.
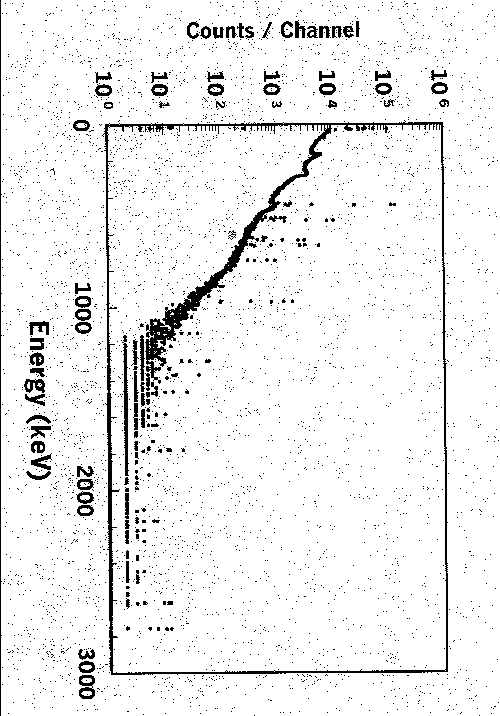
129I has long been considered a potentially problematic radionuclide in low-level radioactive waste disposal4. As a result considerable emphasis has been placed on 129I analyses on the wide variety of low level radioactive waste streams generated at the Savannah River Site. Currently SRTC uses a variant of the silver iodide precipitation method prescribed for EPA drinking water analyses5. As the sample matrix varies widely for the various waste streams, the potential for interferences using a gravimetric-based chemical yielding approach is substantial. The SRTC NAA facility is ideally suited for yielding the stable iodide recoveries for these separations. A filter containing the sample’s AgI precipitate is first analyzed for 129I’s 37 keV gamma ray with a large-area low-energy photon spectrometer. Subsequently, the filter is loaded into a polyethylene vial, and is inserted into a rabbit for activation. Irradiation times are short, on the order of ½ hour. A 1000-second gamma analysis (Fig. 4) on the silver salt of ~30-mg of iodide, using a 25% efficient germanium detector yields peak areas on the order of 105 counts.
Site programs also require analyses of 99Tc, another long-lived (2x105 yr), low-energy beta emitting fission product. These analyses play a significant role in developing and testing flow sheets for high level waste vitrification processes. The measurements also support other waste management and environmental programs. As technetium is a fairly volatile element, especially in the pertechnetate form, accounting for variable levels of technetium loss from sample to sample is always a concern. This concern is heightened for waste streams that require aggressive digestions prior to radiochemical separations. There are no stable technetium isotopes for yielding 99Tc. One analysis path for 99Tc includes 99mTc tracer addition for chemical yielding, radiochemical separation by extraction chromatography, tracer activity measurement by gamma-ray spectrometry and 99Tc activity determination by liquid scintillation counting. LSC counting is deferred for about four days to allow decay of the (6-hr) tracer. Although 99mTc is commercially available, 99Tc analysis requests are not routine at our laboratory. That is, the regular commercial shipments - that would be required to maintain a reliable supply of the short-lived tracer - are not justified. In addition, rapid turn-around requirements that often accompany 99Tc analysis requests make it impractical to spot order 99mTc on a sample-by-sample basis. Preparation of 99mTc by neutron activation of ammonium molybdate is the practical choice for SRTC. Technetium is rapidly separated from the molybdate matrix by solvent extraction. 99mTc levels of up to 2x105 Bq have been generated using this pathway.
Separations of several pure beta-emitting lanthanide fission products (147Pm, 151Sm) from other long-lived fission products and actinides were recently studied using extraction chromatography and stepped eluent concentration changes. The separation tests supported environmental programs and high-level waste programs for safe disposal of old defense-program wastes. Sequential low-volume fractions from columns were collected for subsequent beta and gamma-ray spectrometry. Lanthanide radioisotope tracers were prepared for the tests by neutron activation of praseodymium, neodymium, samarium and europium. The gamma-ray-emitting tracers were used as markers for elution fractions. The tracer radionuclides were easy to measure, and they allowed unequivocal identification of specific lanthanide fractions while procedures were being developed.
Another paper6 at this conference discusses experimental results and Monte Carlo modeling of prompt gamma neutron activation analysis (PGNAA) using lower mass 252Cf sources.
The SRTC NAA facility continues to demonstrate the usefulness of low-flux 252Cf sources. Although the facility can not compete with analytical methods such as ICP-MS on the basis of sensitivity alone, the facility provides adequate sensitivity for many applications for samples analyzed in bulk. This is a useful advantage on difficult-to-digest matrices. The facility offers invaluable support in the arena of radiochemical separations. Short-lived radio-tracers are generated at the facility which are spiked into samples to serve as chemical yield agents for elements being separated. In cases where suitable radio-tracers can not be generated, the facility often helps complete radiometric analyses by providing yields through activation and counting of carriers for elements of interest.