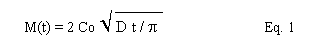
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available to DOE and DOE Contractors from the Office of Scientific and Technical Information, P. O. Box 62 Oak Ridge, TN 37831; prices available from (423) 576-8401.
Available to the public from the National Technical Information Service, U.S. Department of Commerce, 5285 Port Royal Road, Springfield, VA 22161.
The gravimetric weight loss of lead oxide and boric oxide from lead lanthanide borosilicate type glasses was investigated by remelting glass grains in small platinum crucibles at varying temperatures and times. A simple linear equation was developed to describe the volatilization rate of the glass in the crucibles with changes in temperature. The holding time at temperature does not have a very strong impact on the calculated volatilization rate when the time is within a two day period. The volatilization rate was calculated and compared to values obtained from the pilot facility bushing melter off-gas studies at the same temperatures. The calculated volatilization rate from crucibles was approximately twice that measured in the bushing melter off-gas which indicates that either the bushing may have a colder air/glass interface temperature or possibly the off-gas investigations did not capture all of the volatile material.
Special glass compositions and melter systems are being developed by the Savannah River Technology Center (SRTC) to immobilize a stored nitric acid solution containing relatively large quantities of lanthanides and the radioactive isotopes: americium-243 and curium-244. These materials are presently stored in Tank 17.1 at the Separations Canyon in F Area at the Savannah River Site. Immobilization of this material will permit shipment of the material to the Oak Ridge National Laboratory - Heavy Element and Advanced Neutron Facility where it will be transformed by nuclear reactions into other useful transplutonium isotopes. The glass compositions for this program were based on a lead borosilicate optical glass composition developed during World War II by Loeffler.[1] This glass system was initially selected based on its ability to incorporate large quantities of lanthanides and potentially actinides into the glass network structure. These glasses melt sharply at normal glass melting temperatures and have unusually low viscosity making them desirable from a vitrification processing standpoint. Research has shown that increasing levels of lanthanide oxides acts as a flux or softening agent and reduces the melt viscosity.
One melter system under development at SRTC is a closed, platinum rhodium alloy box; heated by passing large electrical currents through the platinum alloy (resistance heat). This type of system was initially developed for use in remelting glass marbles for the manufacture of continuous glass fibers[2] and an extensive body of patent and journal literature exists for this area of glass manufacture. This commercial technology was modified and is being further developed for remote operation with the lead-lanthanide borosilicate glasses using a coupled liquid-frit feed stream.[3] This modified remelt bushing system may be employed for the remote melting and casting of the Am/Cm glass into small cans in the Separations Canyon in F Area.
The volatilization of lead oxide from the Loeffler glasses was considered early in the development of the laboratory process hazards review. One of the major concerns was the volatilization of lead compounds from the glass melt to the off-gas system and the hazards this might cause for personnel working with the system. The findings of J. Matousek [4] were employed to provide an initial estimate of the volatility of lead from a typical alkali lead silicate glass. A rough order of magnitude, estimate of 20 mg/sq.cm/hr for the volatilization at 1400°C of the lead oxide was chosen based on an alkali lead glass containing about 20 wt% lead oxide.
It has been shown [5-7] that the volatilization of lead oxide is significantly increased by increasing temperature, but the rate of volatilization decreased with increasing soak time. Most authors also agree that under equal conditions, the volatilization of lead increases with the fraction of lead in the glass or with reduced SiO2 content in the glass. The volatilization products from lead borosilicate glasses consist mainly of lead oxide while other volatile constituents, e.g. alkali and boron compounds, begin to volatilize at temperatures above 1200°C but at lower rates than the lead oxide. Volatilization appears as a complex process of physical chemistry, in which three simultaneous sub-processes may take part in series:
a. the volatile component diffuses through the melt to the surface of the
glass,
b. the volatile component evaporates from the surface of the melt, and
c. the diffusion of the volatile component through the gas phase from the melt
surface into the adjacent gaseous phase.
Diffusion in the gaseous phase is usually assumed to proceed much faster than the other processes, a and b, which then places these processes (a & b) as the rate controlling processes. The total loss of the volatile component can be expressed as:
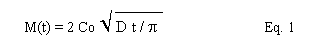
where M(t) is the mass of the volatile component after time t, Co is the initial concentration of the volatile component, and D is the diffusion coefficient of the volatile component. When there is little convection in the glass and temperatures are low, diffusion to the surface should be rate controlling. As temperatures increase, surface evaporation will have more influence and may dominate at extremely high temperatures. In general, the amount of lead vaporized should therefore be proportional to the square root of time. The diffusion coefficient and evaporation coefficient should be related to the reciprocal of temperature expressed in degrees Kelvin. While these theoretical relationships deserve consideration, the experimental conditions found in the present study may be too complicated for such simple relationships. The work described in this report was generally considered scoping in nature due to the complexity of relating small-scale crucible melts to large-scale processing systems.
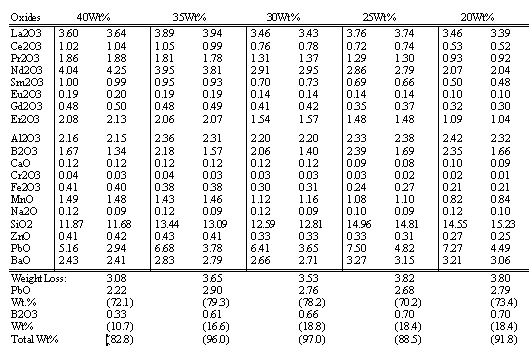
Approximately 1650 grams of simulated Tank 17.3 solids* prepared from reagent grade chemicals. The Tank 17.3 solids were then mixed with the B2000 glass frit** in the proportions of 40, 35, 30, 25, and 20 wt% Tank 17.3 solids. The one kilogram powder mixtures were mixed by thorough shaking and tumbling in 2 liter plastic containers for about ten minutes prior to melting. The calculated chemical compositions of the frit, the 17.3 solids, and the resulting glasses are presented in Table 1.
Table 1. Calculated Chemical Composition of B2000 Glass Frit, Tank 17.3 Solids and Resulting Glasses.
Frit |
17.3 Simulant |
Tank 17.3 Loading |
|||||
La2O3 |
7.12 |
11.23 |
8.76 |
8.56 |
8.35 |
8.15 |
7.94 |
Ce2O3 |
12.90 |
5.16 |
4.52 |
3.87 |
3.23 |
2.58 | |
Pr2O3 |
12.90 |
5.16 |
4.52 |
3.87 |
3.23 |
2.58 | |
Nd2O3 |
27.35 |
10.94 |
9.57 |
8.21 |
6.84 |
5.47 | |
Sm2O3 |
6.34 |
2.54 |
2.22 |
1.90 |
1.59 |
1.27 | |
Eu2O3 |
1.25 |
0.50 |
0.44 |
0.38 |
0.31 |
0.25 | |
Gd2O3 |
3.22 |
1.29 |
1.13 |
0.97 |
0.81 |
0.64 | |
Er2O3 |
12.90 |
5.16 |
4.52 |
3.87 |
3.23 |
2.58 | |
Al2O3 |
6.04 |
0.64 |
3.88 |
4.15 |
4.42 |
4.69 |
4.96 |
B2O3 |
9.20 |
5.52 |
5.98 |
6.44 |
6.90 |
7.36 | |
CaO |
0.03 |
0.01 |
0.01 |
0.01 |
0.01 |
0.01 | |
Cr2O3 |
0.26 |
0.10 |
0.09 |
0.08 |
0.07 |
0.05 | |
Fe2O3 |
2.19 |
0.88 |
0.77 |
0.66 |
0.55 |
0.44 | |
K2O |
0.08 |
0.03 |
0.03 |
0.02 |
0.02 |
0.02 | |
MnO |
8.34 |
3.34 |
2.92 |
2.50 |
2.09 |
1.67 | |
Na2O |
0.28 |
0.11 |
0.10 |
0.08 |
0.07 |
0.06 | |
NiO |
0.09 |
0.04 |
0.03 |
0.03 |
0.02 |
0.02 | |
SiO2 |
44.23 |
26.54 |
28.75 |
30.96 |
33.17 |
35.38 | |
ZnO |
0.01 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 | |
PbO |
24.38 |
14.63 |
15.85 |
17.07 |
18.29 |
19.50 | |
BaO |
9.02 |
5.41 |
5.86 |
6.31 |
6.77 |
7.22 | |
Total |
99.99 |
100.01 |
100.00 |
100.00 |
100.00 |
100.00 |
99.99 |
The materials from Table 1 were melted in a small platinum-rhodium bushing melter. The mixtures were ramped up to 1475°C and additional batch added to bring up the glass level. Problems were encountered with melting and refining of the 20 and 25 wt% Tank 17.3 loaded glasses and only half of the one kilogram batch was melted. All of the available batch for the higher loaded glasses was melted. After melting for two hours at 1475°C the bushing temperature was dropped to about 1400°C and the glass drained.
* Feed Material currently in Tank 17.3 will be retrieved and washed according to a specific pretreatment option. The pretreated feed is to be stored in Tank 17.3
** Frit B2000 was supplied by Ferro Corporation - Cleveland, Ohio.
The glasses were observed to be quite fluid when they were drained into water. Samples of the "as fabricated" glass were submitted to an analytic laboratory for determination of the chemical composition by Inductively Coupled Plasma (ICP) chemical analysis.
Two distinct approaches were taken to investigate volatilization of the Tank 17.3 - B2000 system. The first approach involved simple gravimetric studies of all of the glasses (i.e., various 17.3 loadings) held under isothermal conditions for 48 hours (in addition to the 2 hours at 1475°C during initial fabrication) at temperatures ranging between 1200 to 1450°C. Based on mass loss alone, one would not be able to tell the volatile species in this system. Therefore, the heat treated samples were submitted for chemical analysis by ICP and were compared to the "as-fabricated" compositions. The second approach involved heat treating only the 40 and 35 wt% Tank 17.3 loaded glasses at 1300 and 1400°C for varying lengths of time. The objective and general experimental technique for each approach follows.
The objective of this initial task was to gain insight into the volatilization as a function of temperature for the Tank 17.3 - B2000 system for a given time period. Temperatures ranging from 1450°C (the nominal operating temperature of the bushing melter for the B2000 system) to 1200°C were evaluated. The lower limit was chosen based on volatilization data from the literature dealing with lead-silicate glasses. The amount of dry glass grains placed in the weighed platinum crucible was between 40 and 50 grams. The degree of volatilization was measured by weight loss for the Tank 17.3 series of glasses. An experimental design, as shown in Table 2, was selected for this temperature - waste loading investigation.
Table 2. Experimental Design No.1 - Volatility After 48 Hours
The open surface area in the platinum crucibles was calculated to be about 12.57 square centimeters. Upon removal from the furnace the samples were cooled to room temperature in a dessicator and weighed again. Total mass loss was calculated as a measure of the degree of volatilization. To obtain semi-quantitative analysis on the volatile species, heat treated samples were submitted for chemical analysis and compared to the "as fabricated" sample compositions. It should be noted that the compositional comparison of the "as fabricated" glasses (1475°C for 2 hours) to the "heat treated" glasses will underestimate the total degree of volatilization. That is, during initial fabrication (melting) of the glasses, some degree of volatilization occurs. The volatilization during fabrication is not accounted for by these comparisons.
Since the initial baseline flow sheet targets a 35 wt% Tank 17.3 loading - B2000 glass, a more detailed evaluation of the effects of time and temperature on volatilization was undertaken.
A second glass (40 wt% Tank 17.3 loading) was also evaluated due to the potential for higher waste loadings and because it appears that the lower waste loading glasses were rather difficult to melt at 1450°C. The "as fabricated" glasses were heat treated for various times and temperatures. The degree of volatilization was determined by gravimetric analysis as in the initial study (approach No.1). The same crucibles were employed for these measurements. This will allow for the compilation of data between the two approaches. Table 3 summarizes the experimental design for this approach.
Table 3. Experimental Design No.2 - Volatility at 1400 and 1300°C with Increasing Time.
Tank 17.3 Solids Loading at Temperature | ||||
1400°C |
1300°C | |||
Time |
40% |
35% |
40% |
35% |
2 |
Y |
Y |
Y |
Y |
4 |
Y |
Y |
Y |
Y |
8 |
Y |
Y |
Y |
Y |
24 |
Y |
Y |
Y |
Y |
|
X |
X |
X |
X |

The glasses after melting were chemically analyzed by ICP and compared to the targeted calculated compositions. The B2O3 and PbO were found to be consistently lower than the calculated values while the other oxides were similar. It is expected that some of the lead and boron volatilized during the melt preparation of the glasses prior to any gravimetric testing. Closer inspection of the lead oxide analyses indicated that the loss during melting was usually less than one wt%.
Samples of all the glasses were dried and weighed and placed into weighed platinum crucibles in a furnace at the selected temperatures (see Table 2). After 48 hours at the temperature, the crucibles were removed, cooled and weighed again. The weight lost due to volatilization was obtained and plotted in Figure 1. This figure is a bar graph showing the average volatilization rate per hour from each of the five glasses with Tank 17.3 loadings at the chosen temperatures. The data point for the 20 wt% loaded glass at 1250°C appears suspect, but all the other data points appear to be acceptable within the limits of this test.
The glass volatilization did not appear to be greatly influenced by the level of the Tank 17.3 loading. Accepted theory would lead to the expectation that the highly loaded Tank 17.3 glasses would be low in volatility as they contain less lead oxide. While the glasses melted at 1400 and 1450°C may show some variation due to the content of lead and boron, it is doubtful that these differences would be statistically significant. It should also be noted that three of the glasses with the lower levels of Tank 17.3 loadings were different in appearance after the heat treatment at 1450°C. There was a slight milky opalescence within the glasses. The high level of PbO volatilization, greater than 10 wt%, may have unstabilized the glass, leading to phase separation or devitrification. The glasses treated at 1200 and 1250°C were examined by x-ray diffraction and some small level of crystallization was detected. The phase identification was extremely difficult but may have been rare earth oxides and lead silicate.

Figure 1. Measured Volatilization Rate from Tank 17.3 Glasses After 48 Hours at Temperature.
The glasses heated at 1400°C for 48 hours were analyzed chemically by ICP and compared to the "as fabricated" glass composition. A comparison was made by a mass balance calculation and this data is presented in Table 4. The numbers presented are the product of the composition in weight percent and the weight of glass used in the test. This calculation clearly indicates that the lead and boron losses were the principle cause for the measured weight loss for the 1400°C samples. Based on the mass balance calculation, barium (the other toxic element of B2000 frit) was not volatile. Further calculations showed that the lead loss was approximately 70 to 80% of the total loss and boron was responsible for perhaps 10 to 20% of the loss. This finding will later be shown to be consistent with off-gas measurements on pilot system.
Table 4. Glass Compositions as Fabricated and after 1400°C for 48
Hours
Attempts to plot this data compared to 1/T (Kelvin) did not exactly meet the
expected linear relationships predicted by simple theory. See Figure 2. There
was a slight curvature to the combined data. However, when the data was plotted
with a "best fit" line the following simple linear equation was obtained:
Average Volatility Rate (mg/sq.cm/hr) = 50.17 - (73,630/Temperature - K) Eq. 2
This equation provides an approximation of the average volatility in mg/sq.cm/hr after a significant time period (48 hours) at the selected temperature in Kelvin. This is the equation for small scale, crucible melts. Use of this equation to estimate the volatility from large systems should be used with caution due to the complexities associated with volatilization.
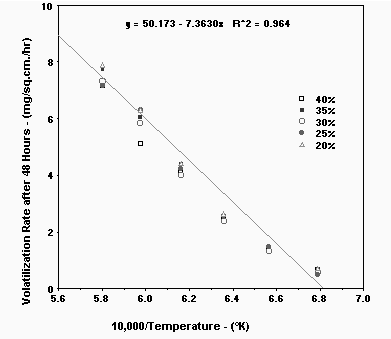
Figure 2. Volatilization Rate of all Glasses as a Function of Temperature.
The glasses with 35 and 40% Tank 17.3 loading were heat treated for time periods between two and twenty-four hours at 1400°C and 1300°C. The resulting weight loss data was combined with the appropriate 48 hour data from Approach No. 1 and is plotted as volatilization weight loss per square centimeter versus time in Figure 3. A continuous almost linear relationship was obtained as shown. It appears that the volatilization at 1400°C is approximately double the volatilization at 1300°C and this is generally supported utilizing Equation 2. This data was also plotted versus the square root of time. The data did not support the square root of time linear relationship which would have identified diffusion as the rate controlling step. It is possible that the low viscosity glasses are experiencing convection at the higher temperatures or other experimental factors could be limiting the utilization of the simple theoretical relationships e.g. the vapor space above the melt is saturated with PbO suppressing volatilization. In addition, the fact that two elements, lead and boron, are both volatilizing may be interfering with the simple explanations of volatility. Again, Equation 2 should provide a reasonable estimate of the volatilization within the temperature range of interest for crucible tests.
A question was raised as to how this measured data compares to the original Process Hazards Review estimate of volatilization. This estimate, of course, was intended to be conservative. The 48 hour average volatilization rate was about one third of the estimate of 20 mg/sq.cm/hr and since Figure 3 approximates a linear relationship this one third ratio would probably hold over the range of times investigated.
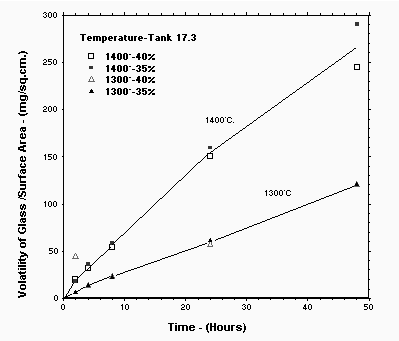
Figure 3. Measured Volatilization per Area as a Function of Time for 35 and 40% Tank 17.3 Loading Glass.
The off-gas system of the Am/Cm bushing melter was tested under a variety of processing conditions using the B2000 glass system.[10] Although six test runs were completed, only the runs where glass was idled at 1450 °C and 1150 °C are comparable to this volatility investigation. Frit and surrogate feed were not fed during these two runs. Additionally, only air was introduced into the off-gas film cooler. The off-gas stream was sampled between the control air inlet and the steam eductor. The sampled stream passed through a cascade impactor and then through an EPA Method 29 sampling train. Filter papers (retention to 0.18 µm) within the impactor were weighed before and after each test run to determine total particulate loading in the off-gas streams.
The compositions of impinger solutions and material retained on the impactor filters were determined by Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES). The ICP-AES was used to measure Pb, Ag, Al, As, B, Ba, Ca, Cd, Ce, Cr, Cu, Er, Eu, Fe, Gd, La, Mn, Nd, Ni, Pr, Se, Sm, and Sr concentrations. Silicon was not measured because the filter papers within the impactor were primarily silicon. Boron results could not be utilized because of the probable boron contamination from the borosilicate glassware. Additionally, high background levels of aluminum and calcium made results for these elements unreliable.
The bushing melter has a glass/air surface area of approximately 177.8 sq. cm. The measured volatilization rates of total emission and PbO were 3.04 mg/cm2/hr and 1.91 mg/cm2/hr respectively while idling at 1450°C.
Thus, PbO accounted for 63 percent of the material lost from the glass surface. Comparison with the crucible studies showed PbO and B2O3 were responsible for 70 to 80 and 10 to 20 percent, of the volatile losses at 1400°C (Approach #1). Considering the major differences in equipment and conditions, these results are very similar. However, the total rates of volatilization from the crucibles at 1450°C were somewhat different. The average total volatility rate at 1450°C (using Equation 2) was 7.44 mg/sq. cm/hr. The rate of volatilization in crucible melts at 1450°C was somewhat more than double the rate in the bushing melter at 1450 °C. Assuming that greater convection currents exist in the bushing melter (due to a larger thermal gradient and the use of forced convection (bubbler), the rate of PbO and B2O3 volatilization should be greater. One possible explanation is the difference between the measured temperature of the bushing melter and the unknown true temperature of its glass surface. Another explanation may be that the off-gas test did not capture all of the volatile material. It is known that some amount of material did plate out on the off-gas film cooler and the off-gas line prior to sampling.
The bushing melter temperature is measured at the outside of the bushing wall. The surface temperature of the glass inside the bushing is not normally measured but is less than the control temperature. A lower measured volatilization rate from the melter may indicate glass surface temperatures below 1400 °C. Until the true temperature of the glass surface is determined, an absolute comparison between the bushing melter and crucible volatilization cannot be made. There was no measurable PbO emission in the bushing melter at the 1150 °C, and total emission was only 0.06 mg/cm2 hr. Extrapolation of crucible results from Equation 2 also predicts a volatilization rate close to zero below 1200°C.
Examination of the data presented in this report provided the following general conclusions for the volatility of PbO and B2O3 from Tank 17.3 -B2000 glass melts:
Due to various problems with glass devitrification with B2000, the composition of the frit for the americium/curium solidification program has been modified.
The information contained in this article was developed during the course of work under Contract No. DE-AC09-89SR18035 with the U.S. Department of Energy.