WSRC-MS-2003-00089, Rev. 1
Demonstration of the UREX Solvent
Extraction Process with Dresden Reactor Fuel
T. S. Rudisill, M. C. Thompson, M. A. Norato,
G. F. Kessinger, R. A. Pierce, and J. D. Johnson
Westinghouse Savannah River Company
Aiken, SC 29808
Introduction
The transmutation of long-lived radionuclides is being developed as part of the Department of Energy’s Advanced Fuel Cycle Initiative to address disposal of commercial nuclear reactor fuel and improve the performance of the geologic repository.[1] The transmutation program will separate the fuel into (1) a transuranium (TRU) product stream for conversion to a mixed oxide reactor fuel, (2) separate technetium-99 (99Tc) and iodine-129 (129I) streams, for conversion to targets for transmutation, and (3) a uranium (U) product stream that meets criteria for disposal as a Class C low-level waste (LLW).
The Plutonium Uranium Extraction (PUREX) process is a mature solvent extraction process for irradiated nuclear fuel that was designed to recover plutonium (Pu) and U. A variation of the PUREX process was conceived to provide the ability to treat large quantities of spent nuclear fuel and to provide the selectivity required for the process. The PUREX process was modified so that only U and Tc are extracted and the TRU isotopes are rejected to the aqueous raffinate with the fission products. This Uranium Extraction process is called UREX.
The goals for the UREX process are to recover >99.9 % of the U and >95 % of the Tc in separate product streams while rejecting >99.9 % of the TRU isotopes to the raffinate. The process must also minimize the waste volume by converting all chemicals to gases during subsequent processing. To meet this requirement, the process was designed to use acetohydroxamic acid (AHA) in the scrub stream which complexes Pu (IV) and Np (IV), preventing them from extracting, and reduces Np (VI) to inextractable Np (V). The test results reported below summarize experiments which demonstrate the UREX process using feed solution prepared from Dresden reactor fuel.[2]
Description of Work
The demonstration of the UREX process was performed using a series of 2 cm annular centrifugal contactors installed in shielded cells at the Savannah River Technology Center. The number of stages in the shielded facility was only sufficient to demonstrate the U/Tc extraction, scrubbing, and Tc stripping portions of the process. Additional contactor stages were assembled in a radiochemical hood to demonstrate U stripping from a portion of the solvent.
Three tests of the UREX process were performed with the feed solution from Dresden reactor fuel. A schematic of the baseline flowsheet is shown on Fig. 1. The 30 vol% tributylphosphate (TBP) solvent was diluted with a (C12 to C16) n-paraffin hydrocarbon with low aromatic content. The feed solution was adjusted to nominally 300 g/L U and 1M nitric acid (HNO3). The U and Tc-bearing solvent was scrubbed with a dilute HNO3 solution containing AHA. The Tc was stripped from the solvent with concentrated HNO3. Circulating cooling water in the extraction and scrub sections maintained the temperature in these stages below 25°C to ensure Tc extraction.
The efficiency of the UREX flowsheet in meeting the process goals was determined by analyzing samples from each product stream. Uranium was stripped from the organic product samples with sodium carbonate and reacidified prior to analysis.
Results and Conclusions
Demonstration of the UREX flowsheet at baseline conditions showed that the process will meet all goals for recovery and decontamination. A comparison of LLW limits and the test results for the U product stream is shown in Table I. The U losses to the Tc and raffinate streams for tests 1 and 2 totaled 0.011 and 0.016 %, respectively. Technetium losses to the U stream were 1.2 and 0.1 % in the two tests; losses to the raffinate were low in both tests demonstrating that >95% of the Tc can be recovered. Uranium and Tc data for test 3 are not available at this time. Loss of Pu and other actinides to the Tc and U product streams was <0.02 % in all three tests of the baseline flowsheet with >99.98 % going to the raffinate.
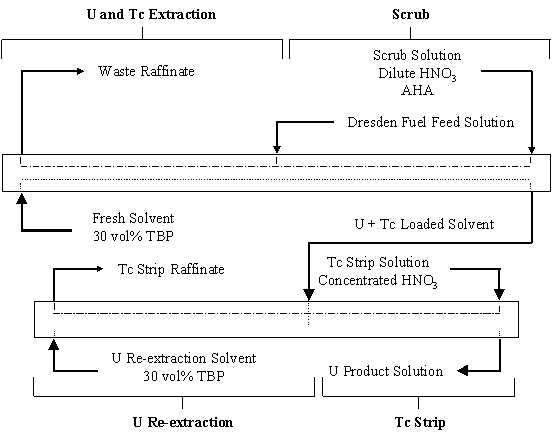
Figure 1. Baseline Flowsheet for UREX Demonstration
Table I. Contamination Levels of U Product Stream
|
Nuclides |
LLW Limits (Ci/m3) |
UREX Test Results(1) |
|||||
|
Class A |
Class B |
Class C |
Test 1 |
Test 2 |
Test 3A |
Test 3B |
|
|
99Tc |
3 |
0.34 |
0.03 |
NA |
NA |
||
|
Nuclides |
700 |
0.05 |
ND |
ND |
ND |
||
|
90Sr |
0.04 |
150 |
7000 |
0.22 |
0.12 |
<0.07 |
<0.07 |
|
TRU Isotopes |
100 h Ci/g |
<296 |
65 |
16 |
29-153 |
||
|
241Pu |
3500 h
Ci/g |
1420 |
214 |
71 |
147-1020 |
||
(1) Assumes the density of bulk uranium trioxide is one-half the crystal density or 3.645 g/cm3.
NA – Not Available
NM – Not Measured
ND – Not Detected
References
- J. J. Laidler, L. Burris, E. D. Collins, J. Duguid, R. N. Henry, E. J. Karell, S. M. McDeavit, M. Thompson, M. A. Williamson, and J. L. Willit, Prog. Nucl. Ener., 38, 65-79 (2001).
- G. F. Kessinger and M. C. Thompson, Dissolution of Dresden Reactor Fuel, WSRC-TR-2002-00448, Westinghouse Savannah River Company, Aiken, SC (2002).