WSRC-MS-2002-00997
Rapid Actinide Column Extraction Methods for Bioassay Samples
S. L. Maxwell,
III and D. J. Fauth
Westinghouse Savannah River Company
Aiken, SC 29808
A new, rapid separation method to assay actinides in urine samples has been developed at the Westinghouse Savannah River Site (SRS). The new method separates plutonium, neptunium, uranium, americium and strontium-90 with high chemical recovery and excellent thorium removal. The method uses calcium phosphate precipitation and stacked TEVA Resin® and TRU Resin® cartridges to separate and purify the actinides. Plutonium and neptunium are separated on TEVA Resin®, while uranium and americium are simultaneously retained and separated on TRU Resin®. Plutonium-236 tracer can be used to allow simultaneous separation and measurement of both plutonium and neptunium using TEVA Resin®. Strontium-90 can also be separated on Sr Resin® by evaporating and redissolving load and rinse solutions collected from the TEVA/TRU column and separating strontium on Sr-Resin®. Fast flow rates are ach
ieved by using small particle size resin cartridges and a vacuum box separation system that will separate 24 samples at a time. This unique approach can be used with urine samples because iron is not present at significant levels in urine and plutonium reduction is accomplished without adding iron (II) to the sample. The advantage of this approach is that actinides can be loaded onto two separate resins in a single load step with simultaneous extraction and assay of neptunium and plutonium with high chemical recovery and excellent removal of matrix interferences.
There have been significant advances in last five to ten years in radiochemical separations, with broad application in a wide range of labs. These improvements in column extraction chromatography have advanced analytical technology in process labs, bioassay labs, and environmental labs. Though these labs are often very different in the sample types analyzed and the analyte levels measured, all have certain commonalities. Sample preparation is required for wide range of analytical work to remove matrix interferences to preconcentrate analytes or remove interferences prior to assay.
Column extraction chromatography has become very popular over the last decade for analytical separations. It offers several advantages over lareg column ion exchange and liquid –liquid solvent extraction. Because extractant-coated resins are often more selective than ion exchange, these new methods are often simpler than older ion exchange techniques. In addition, column extraction methods usually generate less liquid waste, can be employed using lower acid strengths and do not create hazardous organic solvent waste. Recently, the use of vacuum boxes and faster flow rates with smaller particle size resin cartridges has become increasingly popular to reduce separation times. These boxes, which offer flow rates five times faster than gravity flow methods, also allow the operator to apply increased vacuum to any "stubborn" columns which do not flow as fast as others in the batch. With gravity flow, one or more slow columns can slow down the entire batch processing.
In the 1990’s, there was a need to upgrade radiochemistry methods at the Savannah River Site.The Savannah River Site Central Analytical Laboratory (CLAB) replaced a wide range of solvent extraction methods in CLAB used for the previous tweety to thirty years for actinide separation techniques. This has eliminated mixed waste problems caused by the use of solvents such as hexone and thenoly trifluoroacetone (TTA)-xylene. New tandem methods for process and waste analyses were developed and implemented using rapid column extraction chromatography for a wide range of process analyses (1,2, 3).
In 1998 the SRS Bioassay lab also began to upgrade methods analytical methods used in that laboratory. Previous ion exchange methods generated large volumes of acid waste and sometimes resulted in inconsistent tracer recoveries. There was also a need to consolidate actinide analysis for urine into a single sequential method. New methods were implemented to improve the actinide method for urine.
In addition, there was a need to upgrade the method for fecal samples, where total dissolution is difficult, yet very important. A new SRS fecal method recovers actinides from large fecal samples in a small volume of nitric acid with minimal phosphate that can be easily loaded onto small extraction chromatography columns for rapid separation and analysis. This method provides total sample dissolution, high recovery of actinides and excellent purification of plutonium and americium for measurement by alpha-particle spectrometry (4) The new method uses Diphonix Resinâ to extract plutonium and americium from fecal samples that have been ashed and redissolved in dilute hydrochloric acid-hydrofluoric acid solution.
The new urine method takes advantage of stacked resin cartridge technology that separates plutonium, neptunium, americium and uranium using a single column. This method has resulted ina signifciant cost savings in the SRS Bioassay Laboratory.
New Rapid Column Extraction Method for Urine
Monitoring of actinides in urine is an important analysis. A variety of methods have been reported in the literature for measurements of actinides in urine (5). Early solid phase extraction methods tended to use one resin for actinide determination in urine. More recent column extraction methods have used tandem columns to better separate actinide fractions for analysis. Anil Thakker of Eichrom Technologies recently reported a method using UTEVA Resin and TRU Resin to measure uranium, plutonium and americium in urine (6).
In the SRS bioassay laboratory, there was a need to develop radiochemical separation technique that would measure not only plutonium, uranium and americium, but also measure neptunium-237 in urine. The new method needed to provide excellent thorium-228 removal, improve tracer recoveries and consolidate actinide and Sr-90 analyses into a single sample preparation step.
A new stacked column method was developed to meet those needs. The new method separates plutonium, neptunium, uranium, americium and strontium-90 with high chemical recovery and excellent thorium removal. Plutonium and neptunium are separated on TEVA Resin®, while uranium and americium are simultaneously retained and separated on a TRU Resin® cartridge. This unique approach can be used with urine samples because iron is not present at significant levels in urine samples and plutonium reduction is accomplished without adding iron (II) to the sample. The advantage of this approach is that actinides can be loaded onto two separate resins in a single load step with high chemical recovery and excellent thorium removal. Labor costs were reduced significantly and rework has been reduced by 50%. Vacuum boxes and cartridges have allowed much faster analyses than before.
Urine samples were acidified with nitric acid and allowed to stand for two hours. The appropriate tracers (Pu-236, U-232, Am-243) were added to 500 mL aliquots of urine sample. Sr-90 and Np-237 spikes were added to a selected number of samples.
Two drops of 1-octanol and 1 ml of 3M calcium nitrate were added to each sample. Samples were heated on low heat for 1.5 hours and cooled to room temperature. After cooling, 5 mL of 3M ammonium hydrogen phosphate was added to each sample and and the sample was stirred. The samples were adjusted to pH 9 with ammonium hydroxide and the precipitate was allowed to settle for at least one hour. The precipitate and supernate were centrifuged at 3000 rpm for 35 minutes. After decanting the supernate, the precipitate was dissolved in approximately 20 mL of concentrated nitric acid and ashed to dryness on a hot plate at approximately 300-350F. The samples were ashed with 30 wt% hydrogen peroxide several times and then ashed with a mixture of nitric acid and hydrogen peroxide until the residual salts were white.
The evaporated-resin digest was redissolved in the appropriate acid solution for subsequent-column separations. In this work the residues were redissolved in approximately 6 mL of 6M nitric acid. The solution was warmed slightly to ensure complete redissolution and 6 mL of 2.5M aluminum nitrate (previously scrubbed by passing through UTEVA Resin to remove traces of uranium). The final solution contains approximately 12 mLs of 2.5M nitric acid-1.25M aluminum nitrate. Relatively high aluminum nitrate concentrations are used to complex high levels of phosphate in urine samples.
A stacked column method using 2 mL TEVA Resinâcolumns and a 2 mL TRU Resinâ cartridge was employed to isolate the actinides of interest (Figure 1). The TRU cartridge was placed below the TEVA column by luer connection. Pu and Np was retained on TEVA Resin and Am and U on TRU Resin. Ferric ions interfere with americium retention on TRU Resin. Since there are no significant levels of iron in urine, the TRU cartridge can be used in a stacked column with TEVA Resin if the valence adjustment used does not require iron.
The valence of Pu and Np was adjusted to Pu(IV) and Np(IV) by adding 0.5 mL of 1.5M sulfamic acid and 2 mLs of 1.5M ascorbic acid, waiting 3 minutes, and adding 2 mL of 4 M sodium nitrite. After the valence adjustment, the sample solution was loaded onto the stacked TEVA plus TRU column. The TEVA and TRU column was rinsed with 20 mLs of 3M nitric acid to remove matrix components. After the rinsing with nitric acid, the TRU cartridge was removed. To remove thorium from the TEVA column, 3 mLs of 9M hydrochloric acid and 30 mLs 8M hydrochloric acid were added. The Pu and Np were stripped from TEVA Resin with 30 mLs of 0.1M hydrochloric acid-0.05M hydrofluoric acid –0.1M ammonium iodide. Recent work has indicated that plutonium stripping from TEVA resin is improved by using rongalite (sodium formaldehyde sulfoxylate) or titanium chloride as a reductant instead of iodide (7). Four mLs of 0.02M sulfuric acid and approximately 3 mls of 15.7M nitric acid were added to each sample and the sample solution was evaporated. Rongalite is compatible with electrodeposition while titanium is not. Titanium may be used if cerium fluoride microprecipitation is utilized to prepare the alpha mounts. Both are strong reductants and work well in stripping plutonium from TEVA Resin.
Initially, a second-column separation using 1 mL of TEVA Resin was employed to ensure complete removal of all traces of Th-228. It was found that if the U-232 tracer were scrubbed to remove Th-228 daughter that a second TEVA column was not required. To prepare the U-232 tracer, it was diluted in 2 M nitric acid, adjusted to 4M nitric acid, and passed through a TEVA Resin cartridge to remove Th-228. The Th-228 was retained on TEVA Resin and the U-232 passed through the resin. The final solution activity was validated versus a traceable uranium standard using alpha spectrometry. This is performed every 12-18 months.
The americium was stripped from each TRU cartridge using 15 mLs of 4M hydrochloric acid. The uranium was stripped using 20 mL of ammonium bioxalate. To prepare for electrodeposition, solutions were evaporated, wet-ashed using 15.7M nitric acid and 30 wt% hydrogen peroxide, redissolved in a sodium bisulfate matrix and electroplated for 2.5 hours using 0.5 amp current. Additional testing using cerium fluoride microprecipitation was performed using 50 micrograms of cerium in the presence of hydrofluoric acid and filtration and mounting on Gelman 25 mm filters. Solutions prepared for cerium fluoride precipitation did not have to evaporated prior to filtration.
Load and rinse solutions were collected from the TEVA –TRU Resin stacked column, evaporated on a hot plate, and redissolved in 15 mL of 6M nitric acid. Each solution was loaded on to a 2 mL Sr Resin cartridge. The column was rinsed with 15 mLs of 8M nitric acid and stripped with 10 mL of 0.05M nitric acid. The strip solutions were evaporated on planchets that had been annealed in a muffle furnace at 1600°F for 3-1/2 hours in a stainless steel pan. The planchets were cooled and counted for 20 minutes using a gas proportional counter. Sr-90 spikes (205 dpm) were added to blank urine samples to perform Sr-90 yield corrections.
Figure 2 shows tracer recoveries using TEVA Resin to analyze 500 mL urine samples with Pu-242 tracer (1.25 dpm ) added (8). In this initial test performed using TEVA Resin only, ferrous sulfate and ascorbic acid were used to adjust the Pu valence to Pu (III) and sodium nitrite was used to adjust the Pu valence to Pu (IV). The average Pu-242 tracer recovery when cerium fluoride precipitation is used was 102%. The tracer recovery greater than 100% is likely due to alpha counting uncertainty or uncertainty in the tracer value. When samples were analyzed using ammonium iodide in the TEVA strip solution with electrodeposition, a average tracer recovery of 79% was obtained. The lower efficiency of electroplating for these samples may be explained by traces of fluoride that were not completely removed, despite multiple ashing steps with nitric acid and hydrogen peroxide and the addition of 4 mLs of 0.02M sulfuric acid to enhance fluoride volatilization. There may also have been traces of plutonium that were not removed from TEVA Resin.
Figure 3 shows tracer recoveries when rongalite was used in the TEVA stripping solution for a batch of twenty urine samples (9). The average plutonium tracer recovery using rongalite in the strip solution and electroplating was 99%. Since rongalite is a stronger reducing agent than ammonium iodide, the stripping of Pu from TEVA Resin is more effective. In addition, rongalite decomposes into sulfate during the ashing steps and this likely enhances the removal of fluoride ions, which can interfere with electroplating. The amount of bisulfate added in the electrodeposition procedure was reduced to allow for the amount of sulfate resulting from the rongalite.
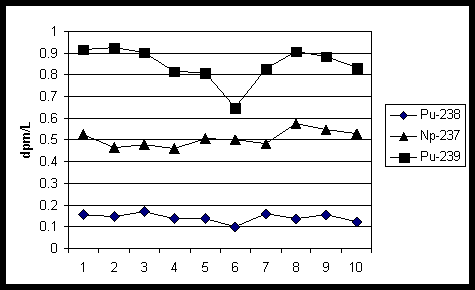
Figure 4 shows tracer recoveries using TEVA Resin to analyze 500 mL urine samples with Pu-236 tracer (0.426 dpm ) added to all samples (10). Ten of the twenty samples were spiked with known amounts of Pu-239 (0.110 dpm/L), Np-237 (0.535 dpm/L) and Pu-238 (0.110 dpm/L). In this test using stacked TEVA and TRU resin cartridges, sulfamic acid and ascorbic acid were used to adjust the Pu valence to Pu (III) and sodium nitrite was used to adjust the Pu valence to Pu (IV). Alpha mounts were prepared using electroplating. The average measured values were as follows: Pu-239 (0.848 dpm/L), Np-237 (0.508 dpm/L) and Pu-238 (0.143 dpm/L). The average bias for Pu-238 and Pu-239 was +30% and +1.0% respectively. The average bias for Np-237 was +5.0%. The larger bias for Pu-238 may have related to the the very low level of Pu-238 standard added.
Figure 5 shows tracer recoveries using TRU Resin to analyze 500 mL urine samples with Am-243 tracer (1.55 dpm) and U-232 tracer (0.554 dpm ) added (11). The average tracer Am-243 recovery was 96.9% and the average U-232 recovery was 84.7% when samples were electroplated.
Figure 6 shows the accuracy achieved on urine samples spiked with Pu, Np, Am, U and Sr (12). The urine samples contained Pu-238 in the range 0.145 to 4.95 dpm/L (N=12), Pu-239 in the range 0.022 to 3.62 dpm/L (N=12), Am-241 in the range 0.55 to 3.5 dpm/L (N=7) , U-234 in the range 0.197 to 2.04 dpm/L (N=4), U-238 in the range 0.147 to 3.07 dpm/L (N=4) and Sr-90 in the range 4.4 to 188 picoCi/L (N=6).
The average bias for Pu-238 and Pu-239 was –14.7% and +12.4% respectively. The average bias for Am-241 measurements was -3.4%. For uranium, the average bias for U-234 and U-238 was +7.8% and +1.5% respectively. The Sr-90 recoveries used to perform yield corrections averaged 90.2%. The Sr-90 bias averaged –4.8% for the spiked samples.
The average bias results are well within the DOELAP bias criteria of –25% to +50%. The average blank values for each radionuclide shown were sufficiently low to be acceptable for SRS bioassay needs.
Conclusion
The stacked cartridge method is a significant advance in the radiochemical analysis of actinides in urine samples. The method provides high chemical recovery, excellent cleanup from interferences such as Th-228, and rapid column flow rates. This unique approach can be used with urine samples because iron is not present at significant levels in urine and plutonium reduction is accomplished without adding iron (II) to the sample. The advantage of this approach is that actinides can be loaded onto two separate resins in a single load step with simultaneous extraction and assay of neptunium and plutonium with high chemical recovery and excellent removal of matrix interferences.
Acknowledgments
This work was performed under the auspices of the Department of Energy, DOE Contract No. DE-AC09-96SR18500. The authors also wish to acknowledge A. Harper Shull, statistician at the Westinghouse Savannah River Co., for his statistical evaluation of the data.
References- Horwitz, E. Philip; Maxwell, S.L. et al., Analytica Chimica Acta, 1995, vol. 310, 63-78
- Maxwell III, S.L. Radioactivity and Radiochemisty, 1997, vol. 8, No 4, 36-44
- Maxwell III, S.L.; Satkowski, J. Radioactivity and Radiochemistry, 2001, vol. 12, No 2, 12-20
- Maxwell III, S.L.; Fauth, D.J. Journal of Radioanalytical and Nuclear Chemistry,2001, vol. 250, No. 1, 117-121
- Alvarez, A; Navarro, N. Applied Radiation and Isotopes, 1996, vol 47, No. 9/10, 869-873
- Thakker, A.H. Journal of Radioanalytical & Nuclear Chemistry, 2001, Vol. 248, No. 2, pp. 453-456
- Maxwell, S.L. and D.J. Fauth, Westinghouse Savannah River Co., unpublished material
- Maxwell III, S.L.; Fauth, D.J. Radioactivity and Radiochemisty, 2000, vol. 11, No 3, 28-24
- Maxwell, S.L. and D.J. Fauth, Westinghouse Savannah River Co., unpublished material Radiochemical Measurements Conference, Nov. 11-15, 2002, Knoxville, TN
- 10. Maxwell, S.L. and D.J. Fauth, Westinghouse Savannah River Co., unpublished material
- 11. Maxwell III, S.L.; Fauth, D.J. Radioactivity and Radiochemisty, 2000, vol. 11, No 3, 28-24
- 12. Maxwell III, S.L.; Fauth, D.J. Radioactivity and Radiochemisty, 2000, vol. 11, No 3, 28-24
Figure 1. Stacked TEVA +TRU Resin Column on Vacuum Box
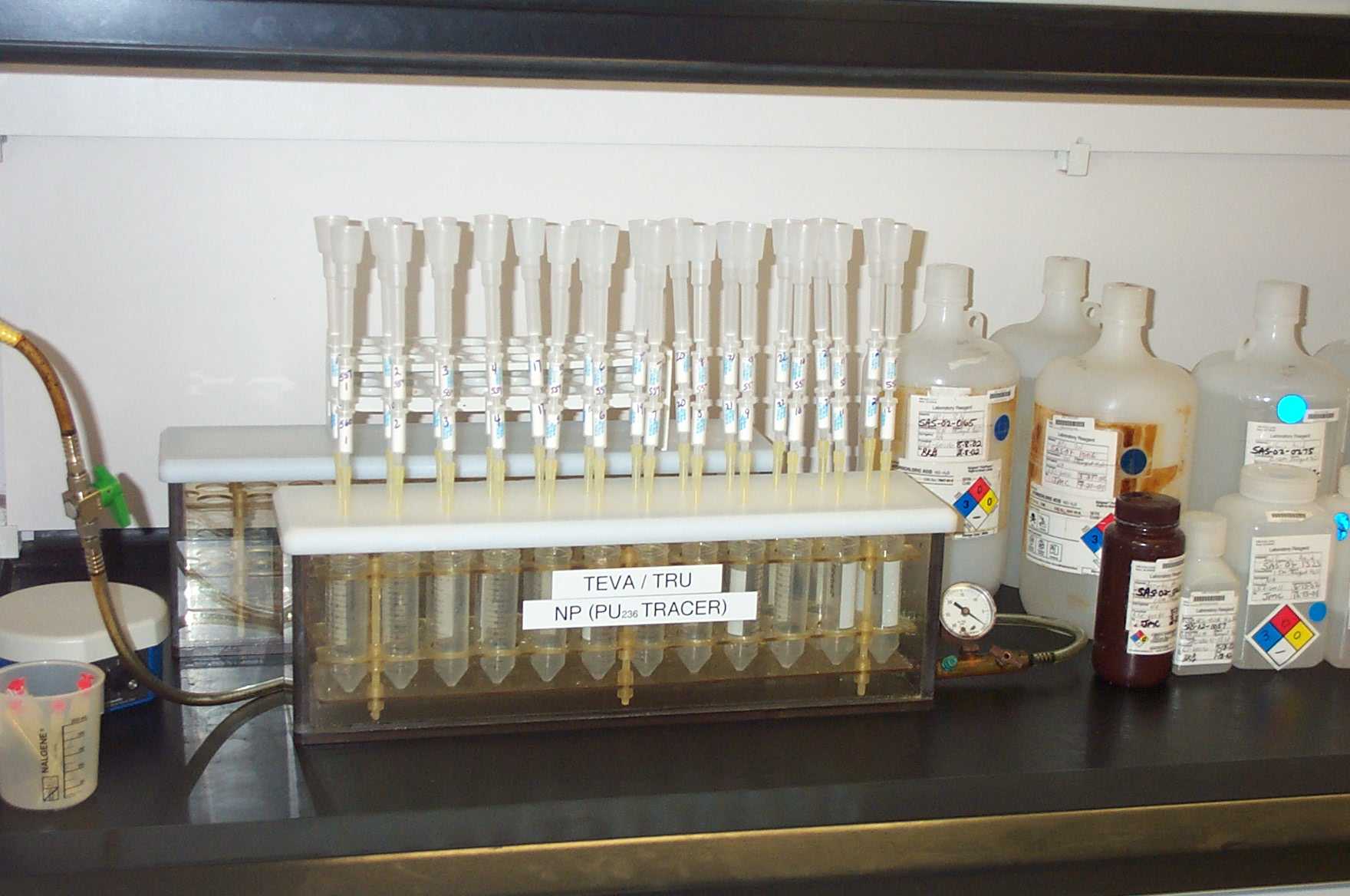
Figure 2. Pu-242 Tracer Recoveries on TEVA Resin
|
Pu-242
Recovery |
Pu-242
Recovery |
|
110.0 |
84.4 |
|
93.3 |
72.2 |
|
92.6 |
69.3 |
|
95.2 |
69.6 |
|
101.5 |
79.8 |
|
99.3 |
84.5 |
|
97.7 |
79.1 |
|
115.4 |
85.5 |
|
107.9 |
84.8 |
|
106.8 |
77.0 |
|
101.6 |
82.5 |
|
102.6 |
|
|
Avg. = 102.0% (± 7.0 @1s) |
Avg. = 79.0% (± 6.2% @1s) |
Reproduced with permission from reference 8. Copyright 2000 Caretaker Communications.
Figure 3. Pu-236 Tracer Recoveries on TEVA Resin
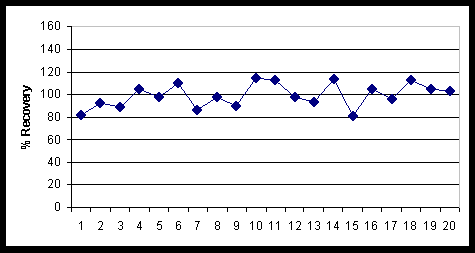
Figure 4. Pu and Np Results on Spiked Urine Samples
| Known values: | Avg. measured: |
| Pu-238 =0.110 dpm/L | Pu-238 =0.143 dpm/L |
| Pu-239 =0.857 dpm/L | Pu-239 =0.848 dpm/L |
| Np-237=0.535 dpm/L | Np-237=0.508 dpm/L |
Figure 5. Am and U-232 Tracer Recoveries on TRU Resin
|
Am-243 |
U-232 |
|
93.2 |
97.9 |
|
92.1 |
74.1 |
|
107.4 |
85.6 |
|
70.3 |
102.9 |
|
102.4 |
90.6 |
|
103.0 |
83.1 |
|
100.2 |
57.7 |
|
103.3 |
81.0 |
|
102.6 |
80.4 |
|
94.7 |
93.3 |
|
Avg. = 96.9% (± 10.6 @1s) |
Avg. = 84.7% (± 12.9 @1s) |
Figure 6 . Accuracy on Spiked Urine Samples
|
Pu-238 |
PU-239 |
Am-241 |
Sr-90 |
|
|
Levels |
0.145-4.95 |
0.022-3.62 |
0.55-3.5 |
5.4-188 |
|
No. |
N=12 |
N=12 |
N=7 |
N=6 |
|
Avg bias |
-14.7% |
+12.4% |
-3.4% |
-4.8% |
|
Banks |
0.016 (N=8) |
0.012 (N=8) |
0.007 (N=23) |
-0.05 (N=4) |
|
U-234 |
U-238 |
U-235 |
||
|
Levels |
0.197-2.04 |
0.147-3.02 |
**** |
|
|
No. |
N=4 |
N=4 |
**** |
|
|
Avg bias |
+7.8% |
+1.5% |
**** |
|
|
Blanks |
0.008 (N=12) |
0.0012 (N=10) |
-0.001 (N=12) |
|
|
DOELAP criteria (Bias: -25% to +50%) |
||||