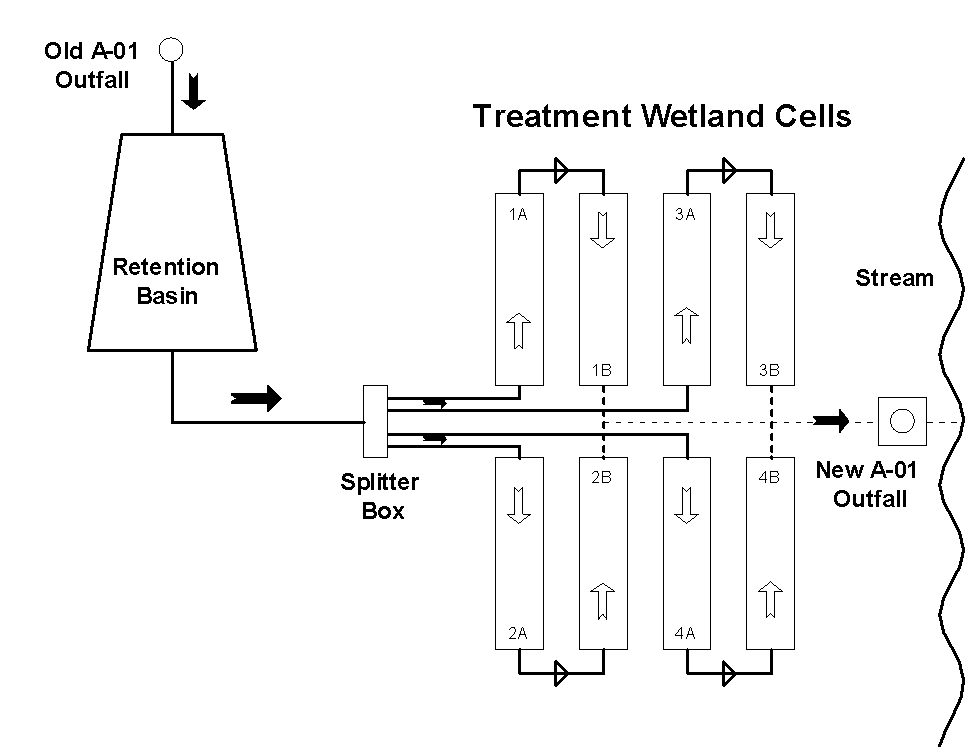
Figure 1. Schematic Diagram of Wetland Treatment System.
WSRC-MS-2002-00600
Constructed Wetlands for Removal of
Heavy Metals from NPDES Outfall Effluent
Eric A. Nelson, Winona L. Specht, James A. Bowers, and John
B. Gladden
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from:
U.S. Department of Commerce, National Technical Information Service, 5285
Port Royal Road, Springfield, VA 22161,
phone: (800) 553-6847,
fax: (703) 605-6900,
email: orders@ntis.fedworld.gov
online ordering: http://www.ntis.gov/help/index.asp
Available electronically at http://www.osti.gov/bridge
Available for a processing fee to U.S. Department
of Energy and its contractors, in paper, from:
U.S. Department of Energy, Office of Scientific and Technical Information, P.O.
Box 62, Oak Ridge, TN 37831-0062,
phone: (865 ) 576-8401,
fax: (865) 576-5728,
email: reports@adonis.osti.gov
Abstract
The A-01 NPDES outfall at the Savannah River Site receives process wastewater discharges and stormwater runoff from the Savannah River Technology Center. Routine monitoring indicated that copper concentrations were regularly higher than discharge permit limit, necessitating treatment of nearly one million gallons of water each day plus storm runoff to meet compliance standards. A conceptual design for a constructed treatment wetland was developed as the most cost-effective alternative. A pilot study was conducted using mesocosms to confirm that the design concept would reduce copper to acceptable levels. After treatment in the mesocosms, effluent copper concentrations were routinely below permit limits, even though the influent concentrations varied widely. During the research phase the wetland system was also effective at reducing total and dissolved mercury. Constructed in the summer of 2000, the treatment system consists of four pairs of one-acre (0.4 hectares) wetland cells (eight acres (3.24 hectares) total) with water flowing from one cell to a second cell, then to the discharge point. The cells are vegetated with Scirpus californicus, and water retention time in the system is approximately 48 hours. Results of the full-scale system during the operation start-up phase have mimicked those generated by the mesocosm study. Copper concentrations at the effluent discharge are often below detection limits (10 µg/L), and total mercury concentrations are reduced throughout the water path through the wetland system. Copper removal efficiency was excellent from the start-up of the system, while mercury efficiency has improved with maturation of the treatment cells. Vegetation development within the cells has been excellent, surpassing 2.5 kg/sq. meter of dry aboveground biomass. Operation and maintenance of the system is minimal, and mainly consists of checking for growth of the vegetation and free flow of the water through the system. This has provided a low cost construction option and low cost maintenance program for the effective treatment of large volumes of permitted water discharges from an industrial area.
Introduction
The ability of natural wetlands to improve many aspects of water quality has been recognized for many years. This natural process has been utilized in many different forms and applications to use constructed treatment wetlands for the purpose of water quality improvement (Moshiri, 1993; Kadlec and Knight, 1996). One aspect of the natural wetland functions that has been capitalized on is the biogeochemical cycling and storage processes that occur in the systems. Heavy metal retention by constructed and natural wetlands has been effectively used in mining regions of the U.S. and Europe to reduce levels of Cu, Zn, Ni, Pb, and other metals in runoff and drainage (Mays and Edwards. 2001). They are equally effective at treating stormwater runoff with high metal concentrations (Walker and Hurl, 2002).
The A-01 NPDES outfall at the Savannah River Site receives process wastewater discharges from the Savannah River Technology Center (SRTC), Savannah River Ecology Laboratory and a few other facilities. Additionally, the outfall receives stormwater runoff from all of these areas. The South Carolina Department of Health and Environmental Control (SCDHEC) has established concentration limits for a variety of chemicals that could be contained in this water. Routine monitoring indicated that copper concentrations were regularly higher than the permit limit and the water routinely failed biomonitoring tests. Other chemicals (e.g. lead, chlorine, mercury, etc) were occasionally higher than the anticipated permit limits. Overall, the largest problem appeared to be the elevated copper levels in the water. A series of studies revealed that the copper was coming from a wide variety of sources and was even elevated in stormwater runoff. The end result of these analyses was that nearly one million gallons of water needed to be treated routinely each day, and during storms up to 20 million gallons would need to be treated.
Westinghouse Savannah River Company (WSRC) personnel explored numerous options to bring the outfall waters into compliance with the permit conditions. The analysis was complicated by the need to treat the large volume of stormwater that contained elevated copper concentrations. Conventional treatment systems for metal removal (e.g. ion exchange, chemical precipitation, etc.) proved to be very expensive for the volume of water that needed to be treated and the extremely low concentrations that must be achieved in the water before it was released to the stream. The search for more cost-effective alternatives resulted in constructed wetlands being considered as an alternative. Constructed wetlands are widely used to treat both domestic and industrial wastewater and have been effective in treating metal containing waters from acid mine drainage. Preliminary evaluations showed that a wetland system might achieve the required level of treatment at the lowest cost for construction and operation.
Methods and Materials
The Treatment Facility. The design provided for a stormwater retention basin that would be used to manage the amount of water going to the wetland treatment cells, moderating the effects of stormwater surges and providing additional water to keep the wetland flooded during dry periods. The treatment cells consisted of four pairs of one acre (0.4 hectare) wetland cells with water flowing from one cell to a second cell, then to the discharge point (Figure 1). Each cell is designed to hold the water for 24 hours, with a 48-hour treatment time for water moving through the system. The cells have a low permeability bottom to prevent water from seeping down into the groundwater, a protective clay layer, and 61 centimeters (24 inches) of hydrosoil in which the plants grow. The hydrosoil is natural, sandy soil amended with fertilizer, lime, organic matter and gypsum. Normal water depth in the cells is maintained at 30.5 centimeters (12 inches) and is variable by removal or addition of gates at the outflow from each cell. The wetland is planted with giant bulrush (Scirpus californicus). Rather than sequestering the metals, the bulrush plants provide a continuing source of organic material to the sediments where bacteria and fungi decompose the plants and maintain anoxic (no oxygen) conditions in the hydric soil. The combined effects of the organic matter and anoxic soil conditions work to capture and immobilize the metals in the soils, with yearly growth, dieback, and decomposition of the plants keeping the soil ecosystem functioning year after year to remove metals and toxicity from the water. Construction of the system began in January 2000, and construction of the entire system was completed in the early fall of 2000. The all wetland cells began receiving continuous water flow for treatment at that time.

Figure 1. Schematic Diagram of Wetland Treatment System.
Sampling and Analysis. Routine monitoring samples are collected at a compositing sampler at the compliance point for monthly reporting. As part of the research effort, monthly grab samples are collected from numerous other locations from the inflow to the system through the discharge to the receiving stream. Water samples are collected from the old compliance point, at the entrance into the wetland cells (after the retention basin), after passage through each of the first wetland cells (A cells), after passage through each of the second wetland cells (B cells), and at the discharge to the stream. Samples are analyzed for copper by method #220.1 and for mercury by the new EPA method 1630 for low level detection of total mercury. All vegetation data were collected from permanent one square meter plots established in the cells during the second growing season (spring 2001).
Results and Discussion
Since the wetland treatment system has been operational, no NPDES permit exceedences of metals limits have been recorded at the compliance point. Metal removal efficiency has improved as the vegetative component of the wetland treatment system established itself.
Copper levels at the old A-01 outfall were above permit limits during 12 of the 17 sampling events to date (Figure 2). Total copper inflow to the system has varied up to 180 µg/L, and copper concentrations at the effluent discharge are often below detection limits (10 µg/L). After passage through the treatment cells, the concentrations during all sample dates were well within permit limits, often falling below detection limits of the analytical procedure. This removal process from the water is occurring very rapidly in the wetland system, with most removal already completed prior to exiting the first cell of the series (A cells in Figure 1, data not reported here). Sediment sampling, which is not reported here, indicated that the majority of the copper is removed in the first half of the initial cell used in the treatment process. This is confirmed by the water chemistry at the exit from the first cell.
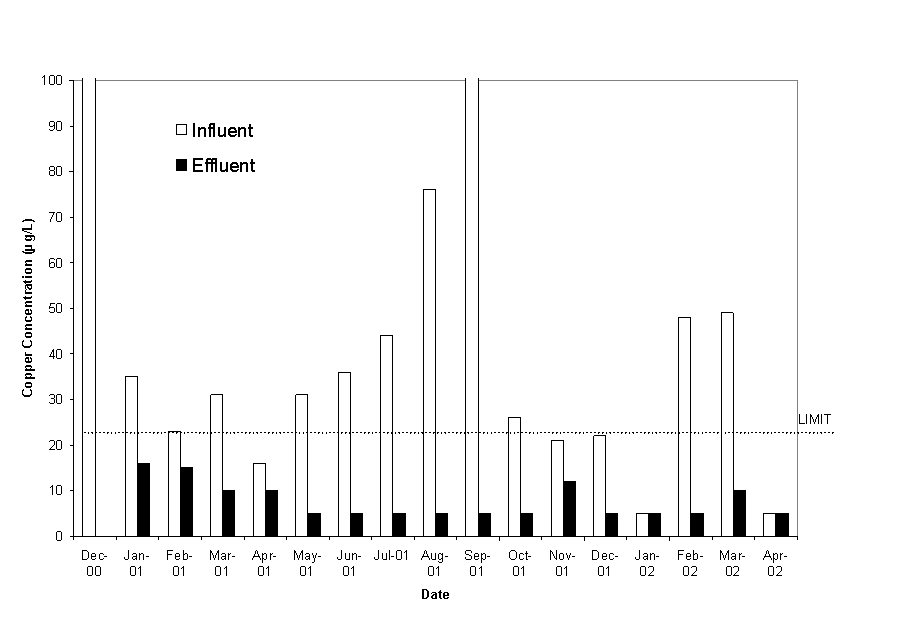
Figure 2. Copper Concentrations in Influent and Effluent Water.
Mercury levels in the water are reduced as the water passes through each phase of the treatment system. The final concentration has been steadily going down as the wetland system has begun to mature in terms of vegetation and geochemistry (Figure 3). During the June 2001 sampling event, the total mercury level was below the permit limit (13 ng/L), and was considerably better than the anticipated mercury reductions from the wetland treatment system during the design phase and initial mesocosm testing. The level has remained below the limit on all sampling dates since and is considered to be associated with the improved efficiency as a result of the complete vegetation establishment and the associated geochemistry of the system. Mercury removal is occurring through the entire water course of the system. Levels after passage through the first cell are typically reduced to 40 percent of the influent value, and reduced by an additional 30 to 40 percent after passage through the second cell in the series. Methylmercury production in the system has shown a seasonal trend, and has been a small component of the total mercury in the effluent. Methylmercury has increased during the warm summer months, but has never exceeded 10 percent of the total mercury at the discharge point.
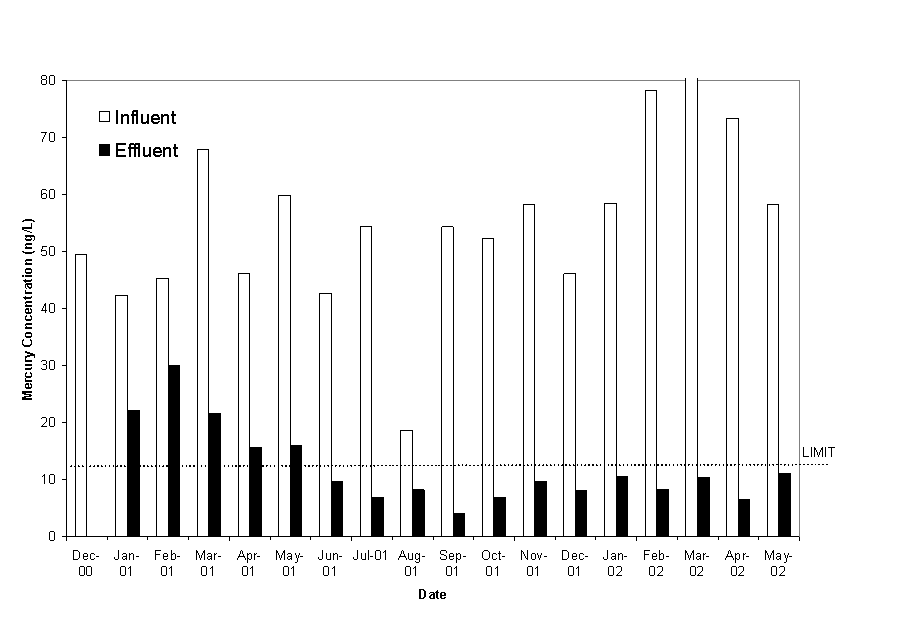
Figure 3. Total Mercury Concentrations in Influent and Effluent Water.
The spread of the vegetation, Scirpus californicus, was very impressive during the second growing season (2001), and continues to perform well during the current year. Many of the cells were planted later in the growing season of 2000 than was scheduled, and did not establish and spread as extensively as expected during the first year. During the second season, however, most plots had increased their number of shoots as much as five-fold. Densities of plots are now near optimal maximums that have been reported in the literature for other species of bulrush. Growth rates for newly emerging shoots above the water have been most impressive. Rates of growth of these new shoots have averaged up to 8 centimeters per day during the most rapid expansion phase, and shoots often surpass 2 meters in length. Aboveground live biomass was harvested after the second growing season, and averaged 2.5 kg/sq. meter of dry material in the wetland cells. This value is above any reported values for Scirpus dominated wetlands (Tanner, 1996). Belowground biomass was not measured.
Conclusions
The wetland treatment system has been very effective in reducing the heavy metal concentrations in the effluent at the A-01 outfall to within permit limits. The single treatment system has brought both metals of concern to within permit limits, and removed toxicity of the discharge. The continuing cost of operation for the facility will be very low, since the system is entirely passive and only requires periodic observation to assure that there is no resistance to normal gravity flow of the water. The wetland is a self-maintaining system through the annual production of organic matter that will renew binding sites for metals and maintain redox conditions for sediment chemistry to continue. This solution has provided a low cost construction option and low cost maintenance program for the effective treatment of large volumes of permitted water discharges from an industrial area. Additional research is being conducted to understand the sediment chemistry and sulfur metabolism, the metal loading and fate within the system, the vegetative cycle of organic matter production and decomposition, and seasonal variations in water quality parameters and chemistry.
Acknowledgements
This research was conducted and the document was prepared in connection with work under U.S. Department of Energy contract DE-AC09-96SR18500.
References
Kadlec, R. H. and R. L. Knight (Eds.). 1996. Treatment Wetlands. Lewis Publishers, Boca Raton, FL.
Mays, P. A. and G. S. Edwards. 2001. "Comparison of Heavy Metal Accumulation in a Natural Wetland and Constructed Wetlands Receiving Acid Mine Drainage." Ecological Engineering 16:487-500.
Moshiri, G. A. (Ed.). 1993. Constructed Wetlands for Water Quality Improvement. Lewis Publishers, Boca Raton, FL.
Tanner, C. C. 1996. "Plants for Constructed Wetland Treatment Systems – A Comparison of the Growth and Nutrient Uptake of Eight Emergent Species." Ecological Engineering 7:59-83.
Walker, D. J. and S. Hurl. 2002. "The Reduction of Heavy Metals in a Stormwater Wetland." Ecological Engineering 18:407-414.