WSRC-MS-2002-00146
Novel Method for Removing Gadolinium from
Used Heavy Water Reactor Moderator
E. Wilde and C. Berry
Westinghouse Savannah River Company
Aiken, SC 29808
M. Goli
Mississippi Valley State University
14000 Highway 82 West
Itta Bena, MS 38941
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/index.html
Available electronically at http://www.osti.gov/bridge
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Keywords: Gadolinium, Nitrate Heavy Water
Abstract
A novel process to treat used heavy water moderator (D2O) contaminated with high concentrations of the neutron poison, gadolinium nitrate,Gd(NO3)3, is described. Gadolinium is removed by precipitation. The resultant precipitate (GdPO4. 6H2O) represents an extremely rare compound of considerable potential value. The resultant supernatant consisting of residual nitrate (NaNO3 or KNO3) is less toxic and easier to process than the original waste. Thus, the alkali metal waste handling can be done with considerably less environmental concern. This waste can potentially be treated by a combination of electrochemical and biological methods.
I. Introduction
A potentially cost-effective process is described for purifying spent heavy water moderator (D2O) contaminated with high concentrations of gadolinium nitrate, a chemical used as a neutron poison in heavy water nuclear reactor operations. The chemical is used for this purpose because the high neutron absorption cross sections of some gadolinium isotopes make gadolinium salts such as GdNO3 effective in controlling nuclear activity in aqueous systems (1, 2, 3). The proposed clean-up process was developed for the clean-up of numerous drums containing spent moderator water at the Savannah) River Site (SRS) near Aiken, SC. The process has the potential to lower the cost of heavy water purification by allowing the use of smaller equipment with less product loss and a reduction in the quantity of secondary waste materials produced in comparison with currently known alternative processes such as ion exchange. Furthermore, the recovery and re-use of the rare metal gadolinium has increasing promise because this element is being used with increasing frequency in medical treatment (MRI techniques) (4, 5).
Microbiological studies (6) revealed that growth of microbes is inhibited at gadolinium concentrations between 100 mg/l and 1000 mg/l, but growth can occur at much higher concentrations of other cations such as sodium and potassium. Since gadolinium levels in drums containing spent moderator at SRS were as high as 280,000 mg/l and averaged 80,000 mg/l, the use of algae or other microbes to sequester gadolinium and or nitrate in the absence of other treatment did not seem feasible. This led to an evaluation of the removal of the gadolinium by precipitation prior to nitrate removal.
II. Precipitation Experiments
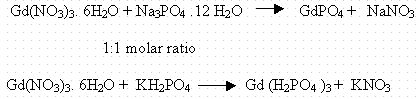
The basic chemical reactions for precipitation with sodium phosphate and potassium phosphate were as follows:

This precipitation step was tested several times with quantities ranging from 100 mg to 2.0 g of Gd(NO3)3. The reactions were tested with both NaPO4 and KH2PO4. The general experimental process was to place 2 g Gd(NO3) 3. 6 H20 in a 125 ml Erlenmeyer flask and dissolved it in 50 ml DI water. An equimolar Na3PO4.12 H2O (s) or aqueous solution prepared by dissolving the solid in 20 ml of DI water was then added to the Gadolinium solution. A magnetic stirrer facilitated the precipitation. In numerous tests, precipitation was always complete in less than five minutes. To evaluate the exact chemical composition of the precipitate, the reaction mixture was filtered trough a Corning 25934-200 filter system using a vacuum (0.1 torr). Then, 5.0 ml of DI water was added in to the reaction flask and the washings were added on to the precipitate to wash off all of the soluble inorganic material. This step was repeated second time to make sure the precipitate transfer was complete. Finally the precipitate was washed with 5 ml of DI water. The filtrate was transferred to a volumetric flask and diluted appropriately. The sample was then analyzed for Gd and Nitrate concentrations using Ion Chromatography. The white gel type precipitate was transferred onto a previously weighed watch glass. The watch glass with the precipitate was heated to dryness in a vacuum oven over night at 300oF (148oC). A yield of >99.9% gadolinium was achieved. The compound is hygroscopic. To determine the extent of water captured in the precipitation process, the precipitate was sent to EAC Elemental Analysis Corporation, Lexington, KY and analyzed for Gadolinium, phosphorus, and oxygen using Proton Induced X-ray Emission (PIXIE). Table 1. Shows the experimental values compared to the theoretical values for the hexahydrate form of the compound. The experimental and theoretical numbers based on GdPO4. 6H2O are, % Gd 48.24 (43.65), P 8.29 (8.38), and O 43.47 (44.41). The experiments using potassium phosphate or sodium phosphate as the precipitating agent demonstrated that virtually all dissolved gadolinium can be precipitated in a single precipitation step that takes minutes and can be done by a solid or liquid (solution) form of the precipitating agent. The yield was close to 100% in all cases. Analyses of the precipitate indicated that it resembles a compound like GdPO4.6H2O. Although it appears that a precipitation step results in a small loss of heavy water due to hydrate formation within the precipitate, that heavy water can be recovered by heating the hydrate to 150°C and condensing the vapor.
III. Conceptual Process for Reprocessing Used Moderator Water
During the course of the developmental work involving potential reuse of spent moderator fluid at SRS, a novel conceptual process for the purification of heavy water containing large concentrations of gadolinium nitrate was developed (Figure 1). The process contains the following steps:
Step 1 has been demonstrated experimentally. Step 2 appears to be achievable using conventional electrochemical procedures. Step 3 has been partially demonstrated experimentally (6) and step 4 is common practice at SRS. Step 5 has not been tested at this time. However, it involves recovery of a potentially highly profitable process byproduct that could greatly improve the overall economics of spent moderator purification and reuse.
The proposed process has three significant advantages over the "baseline" technology, ion-exchange: (1) Gadolinium can be recovered for re-use or potentially sold as a rare, potentially valuable new commercial product, rather than being included in the secondary waste stream, (2) less dilution of heavy water occurs in the process resulting in improved economics when the purified heavy water is reused in nuclear reactors, and (3) secondary waste is reduced more than three-hundred-fold (from approximately 300% of the original volume to <1% of the original volume) in comparison with ion exchange. Step 1 Additional process development work is needed to put this conceptual process into practice.
Acknowledgements
The authors are grateful to Marilyn Frank, Mary Anne Johnson, Fatina Washburn, Sherold Johnson, Mike Polochko, Robert Ray and Charlie Varner for providing technical assistance on this project. Funding for the work was provided under Contract DE-AC09-96SR18500 with the U.S. Department of Energy.
References
Table I. Elemental Analysis of the Precipitate
from the
Reaction of Gadolinium Nitrate with Potassium Phosphate
|
Element |
Measured Value (%) |
GdPO4.6H2O
|
|
Gadolinium |
48.24 |
43.65 |
|
Phosphorus |
8.29 |
8.38 |
|
Oxygen |
43.47 |
44.41 |
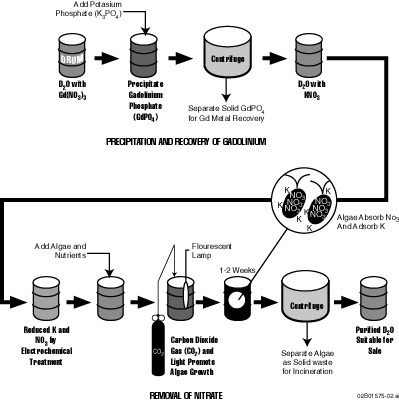
Figure 1. Conceptual Process for Purifying
Drums of Heavy Water Containing Gd(NO3)3