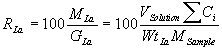 (1)
(1)WSRC-MS-2001-00451
Demonstration of the Feasibility of Recovering Americium and
Curium Isotopes from a Lanthanide Borosilicate Glass
T. S. Rudisill, D. K. Peeler, and T. B. Edwards
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/index.html
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Abstract
A solution containing kilogram quantities of highly radioactive isotopes of americium and curium (Am/Cm) is currently stored in a process tank at the Department of Energy’s Savannah River Site. This tank and its vital support systems are old, subject to deterioration, and prone to possible leakage. To address the stabilization of this material, vitrification of the isotopes has been considered. Potentially, the glass could be shipped to the isotope production and distribution programs at the Oak Ridge National Laboratory for californium-252 production and use by the transplutonium research community. However, before the Am/Cm could be used in these programs, it must be recovered from the glass.
To demonstrate the feasibility of recovering the Am/Cm isotopes from a glass, a series of small-scale experiments was performed as part of a compositional variability study. Glasses fabricated during the study utilized lanthanide elements as surrogates for Am/Cm due to the high specific activity of these materials. In the dissolution tests, glass formulations representative of potential uncertainties in the composition of the Am/Cm solution were fabricated, ground to a -35 to +60 mesh particle size, and dissolved in 8M nitric acid at 110° C. Under these conditions, at least 98% of the lanthanide oxides in the glass dissolved in less than 2 h meeting a recoverability criterion established for the vitrification process and imposing no limitations on the acceptable glass composition region.
Dissolution of the lanthanide borosilicate glasses was described by a spherical particle model based on the observation that the rate of change of the mass to surface area ratio remains constant. Calculation of dissolution rates using the model showed that the rate was proportional to the lanthanide oxide concentration in the glass. When silicon oxide (SiO2) was replaced with a lanthanide element at higher (simulated Am/Cm) loadings, the glass became more easily dissolved in nitric acid due to the solubility of the lanthanide oxides compared to SiO2.
Introduction
Approximately 15,000 L of solution containing isotopes of americium and curium (Am/Cm) are currently stored in an F-Canyon Facility process tank at the Department of Energy’s Savannah River Site (SRS). The isotopes were recovered during plutonium-242 production campaigns in the mid and late 1970's. The tank inventory includes approximately 11.5 kg of Am/Cm, 11 kg of plutonium and uranium, 76 kg of lanthanide fission products, and 116 kg of other metal impurities [1]. The continued storage of this solution was identified as an item of urgent concern in the Defense Nuclear Facility Safety Board’s (DNFSB’s) Recommendation 94-1 [2]. The storage tank and its vital support systems are old, subject to deterioration, and prone to possible leakage. To address the DNFSB’s concern, stabilization of the material by vitrification has been considered. Prior to vitrification, a series of in-tank processing operations would be performed to separate the Am/Cm, plutonium, and lanthanide fission products from the bulk of the uranium and metal impurities present in the solution and prepare the feed for the vitrification process [1].
Once vitrified, the Am/Cm would be stored at SRS until a decision is made concerning its final disposition. Potentially, the glass could be shipped to the isotope production and distribution programs at the Oak Ridge National Laboratory [3]. Possible uses would include the fabrication of targets for californium-252 production in the High Flux Isotope Reactor and distribution to the transplutonium research community. However, before the Am/Cm could be used in these programs, it must be recovered from the glass.
To facilitate high Am/Cm glass loadings, a lanthanide borosilicate glass formulation containing up to 55 wt% lanthanide oxides was initially selected for the vitrification process. The lanthanide oxides in the glass, as surrogates for Am/Cm, were successfully leached from the simulated glass using 15.7M nitric acid [4]. In a subsequent study, both lanthanide and actinide elements were successfully recovered from a glass containing nominally 1 wt% curium oxide by grinding the glass to less than 200 mesh and dissolving in 15.7M nitric acid at 110° C [5].
During the development of the vitrification process, a compositional variability study was performed to evaluate the effect of uncertainties in the solution composition on the glass processing and product performance properties. The intent of the study was to define an acceptable glass composition region where these properties met predefined acceptance criteria. To ensure that Am/Cm could be recovered from the product glasses, a 98% recovery of the lanthanides (as surrogates for Am/Cm) was required within 2 h when the glass was ground to a -35 to +60 mesh particle size and leached in 8M nitric acid at 110° C [6,7].
Experimental
The fabrication of the surrogate glasses used in the Am/Cm variability study are summarized by Peeler et al. [6,7] To prepare the glasses for the recoverability tests, 60 glasses were initially ground and screened to obtain approximately 2 g of glass with a –35 to +60 mesh particle size. Nominally 0.25 g of the ground glass was transferred into each of 5 clean and dry 50 mL Teflonä vessels. A 10 mL aliquot of 8M nitric acid was then transferred by pipette into each vessel. The vessels were sealed and placed in an oven preheated to 110° C. The first vessel was removed from the oven after 0.25 h. Subsequent vessels were removed at 0.50, 1.00, 2.00, and 4.00 h. Upon removal, each vessel was allowed to cool and the contents were transferred to a 200 mL volumetric flask. The flask was diluted to volume with deionized water. A 10 mL sample of the diluted dissolving solution was removed using a sterile plastic syringe and expelled through a 0.45 m m filter disk into a plastic sample bottle. The samples were analyzed for elements of interest by inductively coupled plasma emission spectroscopy.
Results
The recovery (RLa) of the lanthanide elements as a function of dissolution time was calculated for the 60 glass formulations using the recovered mass (MLa) from the solution analyses expressed as a percentage of the total mass in each glass (GLa) (see equation (1)),
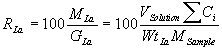 (1)
(1)
where MLa is the product of the volume of solution (Vsolution = 200 mL) and the sum of the measured lanthanide concentrations (Ci), and GLa is the product of the lanthanide mass fraction (WtLa) and glass sample size (MSample » 0.25 g). Recovery data for 3 glasses representative of the range of lanthanide glass loadings are shown on figure 1. These data are typical of the lanthanide recoveries obtained during the dissolution experiments; a complete presentation of the recovery data is provided by Peeler et al. [7].
Figure 1 illustrates that the lanthanide recovery for each of the glasses generally approached 100% at nominally 2 h. However, the recovery for several glass formulations (e.g., 43.0 wt% lanthanide oxide glass loading) was actually slightly less (95-98%) than the acceptance criterion (98%) at 2 h. This small shortfall in recovery was found to be statistically insignificant based on the variance in the measured concentrations used to calculate the masses of the recovered lanthanides and the total lanthanides in the glass. Using a propagation of errors technique, the precision of the estimated lanthanide recoveries was conservatively estimated at ± 3.5% with 95% confidence. Therefore, all glasses met the recoverability criterion established for the variability study and no limitations were placed on the acceptable glass composition region as a result of the recoverability tests.
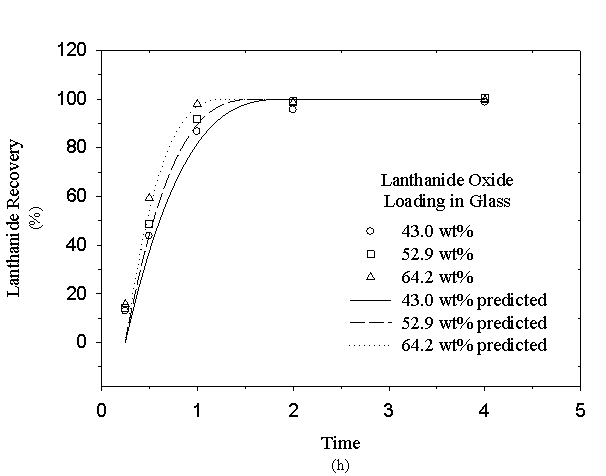
Figure 1. Recovery of Lanthanide Elements from Surrogate Am/Cm Glasses
Recoverability data for the lanthanide elements can be used to calculate a dissolution rate for the surrogate Am/Cm glasses. Rudisill et al. [5] showed that the rate of change of the mass (M) to surface area (SA) ratio with respect to time during nitric acid dissolution was constant (-KR) for a 38 wt% lanthanide borosilicate glass (see equation (2)).
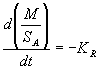 (2)
(2)
This constant, equal to the negative dissolution rate, is calculated from the data by assuming the glass particles can be approximated by spherical geometry and substituting for the mass and surface area in terms of the particle diameter and density (r g). Following integration, with the initial glass particle diameter equal to D0, an equation for the particle diameter (Dt) as a function of time is obtained (see equation (3)).
![]() (3)
(3)
For a glass particle, the lanthanide recovery defined by equation (1) can be approximated using the ratio of the mass of glass at any time to the initial mass prior to dissolution. Substituting for mass in terms of the particle diameter and density yields equation (4) which can be used to calculate the glass dissolution rate from the lanthanide recovery data.
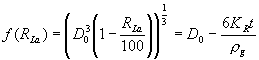 (4)
(4)
Inspection of equation (4) shows that a plot of f(RLa) versus time is linear with a slope of -6KR/r g and a y-intercept of D0. To calculate the dissolution rates, an initial glass particle diameter of 324 m m was used. This value is the geometric mean of a spherical particle which passes through a 35 mesh screen (420 m m) and is retained on a 60 mesh screen (250 m m). The density of each glass formulation was measured by gas pycnometry [6,7].
The measured dissolution rates for the surrogate Am/Cm glasses are plotted as a function of the lanthanide oxide loadings on figure 2. From the figure, one can see that the dissolution rate is generally proportional to the lanthanide oxide concentration of the glass. The lanthanide oxides are soluble in nitric acid [8]. At lower lanthanide loadings when these oxides are replaced with silicon oxide (SiO2) , which is mostly insoluble in nitric acid [9], the glass dissolves at a slower rate. Other factors, such as minor component concentrations in the glass and particle morphology (i.e., available surface area), also contribute to the ease of dissolution; however, for these experiments, the lanthanide oxide loading was the controlling variable. This is consistent with measured glass composition/physical property relationships which were dependent upon the total lanthanide oxide content of the glass [7].
The dissolution rates shown on figure 2 can be used to calculate the expected lanthanide recovery as a function of time. Using equation (3) to calculate the particle diameter at any time,
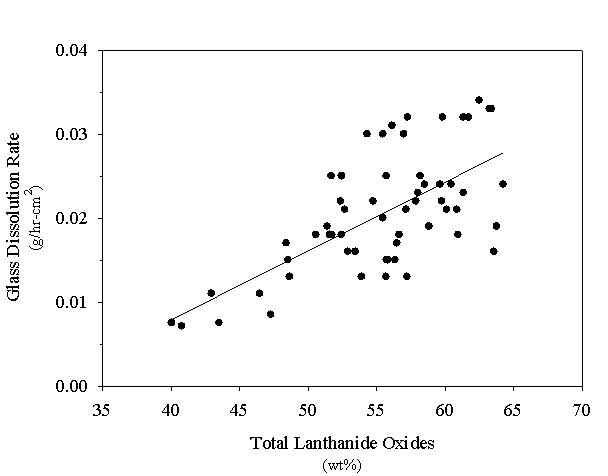
Figure 2. Dissolution Rate of Surrogate Am/Cm Glasses
the lanthanide recovery (RLa) is calculated using the ratio of the mass of glass at any time to the initial mass prior to dissolution. Predictive curves generated using this technique are included for the recovery data plotted on figure 1. It was necessary to shift the origin of the curves by 0.1-0.2 h to account for slower dissolution rates while the nitric acid solution heated to 110° C. This analysis assumes isothermal dissolving conditions. The predictive curves also support the conclusion that all glass formulations meet the recoverability criterion; at least a 98% recovery of the lanthanide elements was predicted in less than 2 h for all glasses which were tested.
Conclusions
A series of lanthanide borosilicate glasses was dissolved to demonstrate the feasibility of recovering Am/Cm isotopes following vitrification. In the dissolution tests, glass formulations representative of potential uncertainties in the composition of the Am/Cm solution were fabricated and dissolved as part of a compositional variability study. After the glasses were ground to a –35 to +60 mesh particle size, dissolution of at least 98% of the lanthanide elements was obtained in less than 2 h when placed in 8M nitric acid at 110° C. For dissolution tests in which the measured lanthanide recoveries were slightly less than 98%, uncertainties associated in the measured glass compositions and the dissolving solution analyses accounted for the difference. Therefore, all glass formulations tested met the recoverability criterion and no limitations were placed on the acceptable glass composition region as a result of the recoverability tests.
Dissolution of the lanthanide borosilicate glasses was described by a spherical particle model based on the observation that the rate of change of the mass to surface area ratio remains constant. Calculated dissolution rates for the glasses showed that the rate was proportional to the lanthanide oxide concentration in the glass. When the nitric acid-soluble lanthanide oxides were replaced with insoluble SiO2 at lower (simulated Am/Cm) loadings, the glass dissolved at a slower rate. Predictive curves developed using the model also supported the conclusion that all glass formulations met the recoverability criterion; since, at least 98% recovery of the lanthanide elements was predicted in less than 2 h.
References