WSRC-MS-2001-00247
A 1600 Liter Tritium Hydride Storage Vessel
J. E. Klein
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Abstract
Titanium was selected for evaluation as a tritium storage material. Titanium-deuterium desorption isotherm data at 550, 600, 649, 700, and 760°C are presented and were used to evaluate storage vessel design loading limits. Two prototype Hydride Storage Vessels (HSVs) containing a nominal 4400 grams of Ergenics HY-STOR 106Ô titanium sponge were tested to determine activation, loading, and desorption conditions. HSV loadings were performed at 5, 7.5, 10, 15, and 20 SLPM. HSV desorptions were done under a variety of vacuum conditions at temperatures up to 700°C. Desorption tests showed a significant amount of gas can be removed from the HSV by desorption, but tritium removal by isotopic exchange will be needed for vessel disposal.
Introduction
At the Savannah River Site (SRS) Tritium Facilities, periodically there is a need for additional tritium storage that exceeds convenient transfers of gas to tanks or process hydride beds. The tritium can be removed from the process and may not be needed for several years. Options include storage in the facility after installing additional process tanks or hydride beds, as a compressed gas in transportable containers or cylinders, or as a portable metal hydride in a container.
Increasing facility process storage capacity for tritium would be expensive and not utilized for normal operations. It was also desirable to remove the tritium from the process building to maintain safety analyses inventory limits. Storage as a compressed gas has the disadvantages of being released if the container fails and the increase in gas pressure from the decay of tritium to He-3. For these reasons it was decided to store excess tritium as a metal tritide. A Hydride Storage Vessel (HSV) has the advantages of compact storage of hydrogen isotopes and its ability to be transported to alternative storage locations.
Several hydrides were investigated for use in the HSV. Titanium was chosen over depleted uranium (DU), a LaNi4.15Al0.85 alloy, and zirconium. DU has the undesirable characteristic of being pyrophoric, slightly radioactive, and does not retain He-3 in the metal matrix as well as other materials. On a per weight basis, titanium has a greater storage capacity than the LaNi4.15Al0.85 alloy and a lower tritium equilibrium pressure. Titanium was chosen over zirconium because the desorption temperature for tritium recovery from titanium is lower than that needed for zirconium. Titanium does have the disadvantage of having a lower desorption pressure than DU or LaNi4.15Al0.85 which inhibits tritium recovery.
Background
Titanium Form
Titanium has been previously proposed as a tritium storage material1,2 and tests with Ti sponge have demonstrated desorption to an atomic ratio of 0.08 H/M.3 Traditional titanium sponge is manufactured by magnesium reduction of titanium tetrachloride. This form of titanium has chlorine greater than the SRS 250 ppm limit. Vacuum evacuation at 900°C can reduce the chloride content of the titanium3, but alternate forms were considered for use.
Two other forms of titanium investigated were the use of commercial grade material cut to desired size and "titanium sponge" manufactured and sold as Ergenics HY-STOR 106Ô .4 Commercial Grade 2 titanium rod, 6.35 mm (1/4 inch) O.D. cut to 6.35 mm (1/4 inch) lengths, was purchased from G&S Titanium and will be referred to as "G&S rod". The cut rod was degreased using a solvent to remove the cutting tool lubricant. Chemical analysis of the material gave composition of 0.01 wt% C, 0.0006 wt% N, 0.06 wt% Fe, 0.14 wt% O, 35 ppm H2, with the balance being titanium. HY-STOR 106Ô is sold as –9.53 to 4.0 mm (-3/8 to + 5 mesh) titanium particles resembling gravel. Technically, HY-STOR 106Ô is not titanium sponge, but textured Ti particles generated by a proprietary process. Impurities totals for the material tested were 0.36 to 0.47 wt% containing various amounts of Al, C, Cl, Cr, Fe, H, Mg, Mn, Mo, N, Ni, O, Si, Sn, and V.
Bench Scale Tests
Bench scale tests were conducted to determine activation conditions needed for hydrogen absorption and to generate desorption isotherm data. Desorption isotherm data has been published by Mueller6, but did not cover the full range of test conditions for this work. Instead of expressing titanium hydrogen capacity in weight percent hydrogen, capacity or loading will be expressed as the atomic ratio of hydrogen isotope atoms per atom of metal: X/M, X equals H, D, or T. For more than one isotope, X/M ratios will represent as Q/M where Q equals H plus D plus T. To calculate the moles of metal, M, a molecular weight of 47.9 grams per mole was used. The nominal upper capacity for titanium is 2.0 X/M.
Activation and deuterium absorption test results for G&S rod samples concluded the Ti rod had difficulty absorbing deuterium at room temperature. Rod gas absorption was very sensitive to gaseous impurities in the deuterium and required additional heating to get significant gas absorption. Post-test inspection of the hydrided rods showed some pieces retained a cylindrical shape, but with appearance of dried/cracked mud, while other pieces broke into many parts and particles. Auger Electron Analysis showed a 25.3 angstroms thick outer oxide layer on the outside of the rod which could be cleaned/reduced to 2.3 angstroms with a mixture of hydroflouric and nitric acid. Similar activation and absorption testing with HY-STOR 106Ô titanium showed the Ergenics material to have better gas absorption properties and was chosen for testing and use in the HSV.
To determine activation conditions for the titanium, a 32 factorial study was performed. Activation temperatures of 400°C, 500°C, and 600°C and vacuum levels of 1.33 x10-1, 1.33 x10-2, 1.33 x10-3 Pa (1x10-3, 1x10-4, and 1x10-5 torr, respectively) were chosen. A 12.7 mm (1/2 inch) O.D. by 0.889 mm (0.035 inch) wall "U-tube" filled with four gram titanium samples were used for the tests. A pressure transducer was connected to one end of the U-tube and the vacuum line connected to the other. The sample was evacuated to 1.33x10-1 Pa and then the furnace controller turned on to its test set-point value. The sample was evacuated at the test temperature until the evacuation pressure was reached or 24 hours had elapsed, whatever came first. The sample was then cooled at least one-half hour which allowed the sample to approach room temperature. The sample was then valved open to a one liter calibrated volume filled with 173.3 kPa (1300 torr) of deuterium to determine gas absorption.
There was some scatter in the results with one difficulty being the change in the zero of the 0.133 Pa (1 torr) transducer when tilted slightly or bumped from its zeroed position. The samples were found to consistently hydride at room temperature to greater than 1.5 D/M if the activation temperature was 500 or 600°C and under a vacuum of 1.33 x10-2 Pa (1x10-4 torr), or lower. In most cases, a vacuum of 1.33 x10-3 Pa (1x10-5 torr) was not reached and activation stopped after 24 hours.
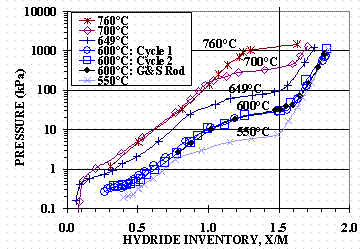
Deuterium desorption isotherms were generated for the HY-STOR 106Ô titanium at 550, 600, 649, 700, and 760°C. A four gram sample in a 10.2 cm (four inch) tall, 12.7 mm (1/2 inch) O.D. by 1.24 mm (0.049 inch) wall 304L stainless steel tube was used for the isotherms. The cycle order for the isotherms was 600, 600, 649, 550, 700, and 760°C. Figure 1 shows the isotherm results for the four gram sample along with a 600°C protium desorption isotherm for a 111 gram sample of G&S Ti rod at 600°C. The vertical line at 1.5 X/M shows the approximate starting location of the d-phase transition region.

Figure 1. Titanium Isotherms
The binary phase diagram for the titanium-hydrogen system7 exists as a combination of the a-phase and the d-phase below circa 300°C, but a b-phase is also present above 300°C. The isotherms in Figure 1 show the d-phase region, the d+b-phase region, the b-phase region, but the b+a region and the a-phase regions are not clearly defined. The rapid decrease in isotherm pressure below 400 Pa (3 torr) for the 700 and 760°C isotherms is attributed to poor mass balance closure due to deuterium permeation out of the sample tube at the higher pressures.
Hydriding and dehydriding of many metal hydrides will cause breakage of the material into small particles or fines, which need to be filtered to prevent contamination of vessel valve seats. Sample #8, a four gram sample of HY-STOR 106Ô which had undergone 16 deuterium hydride cycles, and Sample #11, a four gram sample of HY-STOR 106Ô which had undergone 25 deuterium hydride cycles, had some of their fines analyzed for particle size from SEM micrographs. Measurements of the largest particle dimension where taken and converted to actual size by comparing these dimensions to a reference scale on the micrograph.
Of the 46 particles analyzed for Sample #8, the smallest was 8m m, the only particle smaller than 10m m, the largest was 209m m, and the average was 48m m. 70 percent of the particles analyzed were in the 10 to 60m m range. The 70 particles analyzed for Sample #11 had similar results: the smallest was 7m m, the only particle smaller than 10m m, the largest was 180m m, the average was 50m m, and 67 percent of the particles were in the 10 to 60m m range. These data lead to the use of a 5 mm filter for the HSV.
Experimental
HSV Prototype Vessels
Design criteria selected for an HSV were: 1) a 200-500 grams tritium capacity to limit the number of storage vessels to be fabricated, 2) a storage life of seven to ten years before unloading, 3) a total weight of less than 22.7 kg (50 pounds) so the vessel could be carried by one person without additional hoists or lifts, 4) dimensional limits of less than 38.1 cm (15 inches) in diameter and 63.5 cm (25 inches) in length to allow passage into and out of the glove box air locks, and 5) tritium recovery by either heating the vessel under vacuum or by isotopic exchange.
Two prototypes were assembled for testing. HSV-P1 contained 4399 grams of HY-STOR 106Ô and HSV-P2 contained 4473 grams of HY-STOR 106Ô . A schematic of the HSV prototype design for testing is shown in Figure 2.
The vessels were constructed of a 14.0 cm (5-1/2 inch) long piece of 15.2 cm (six inch) schedule 40S Type TP347H stainless steel pipe, nominal 16.93 cm (6.625 inch) O.D. and 15.41 cm (6.065 inch) I.D, welded to two, 15.2 cm (six inch) schedule 80S Type TP347H stainless steel pipe caps: schedule 40S pipe caps were not available at the time of fabrication. The use of TP347H stainless steel avoids the need for heat treating the vessel after welding.8 A stand to hold the vessel in a vertical position was attached to the bottom and a handle, which also protects the valves, was attached to the top of the vessel. The fill height of the titanium was below the pipe cap weld preventing contamination of the closure weld with titanium. The vessel in either the vertical or horizontal position has only one of the process tube filters covered by the titanium.
The pressure vessel design temperatures and pressures were specified as 7.00 MPa (1000 psig) at 121°C (250°F), 1.43MPa (192 psig) at 760°C (1400° F), and full vacuum at 760°C. The dimensions of the HSV classify it as an ASME pressure vessel, but a waiver to the requirement of a relief device was requested and obtained.
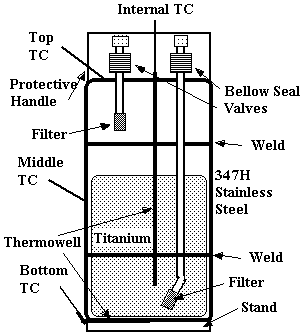
Figure 2. HSV Schematic
The HSV has two valved inlet/outlet 9.53 mm (3/8 inch) O.D. by 1.65 mm (0.065 inch) wall Type 316 or 304 stainless steel process gas tubes. The tubes inside the vessel are fitted with 2.54 cm (one inch) long, sintered 316 stainless steel, 5m m porous filter cups. The one long, or bottom, tube, extends almost to the bottom of the vessel while the other short, or top tube, extends approximately 5.08 cm (two inches) below the top pipe cap. The bottom and top tubes are extended out from the body of the vessel to allow for lower temperatures at the valves than the vessel body during vessel heating. NuproÒ SS-4HS-TW welded, bellow seal valves with StelliteÔ stem inserts were used.
Two external thermowells made from 4.76 mm (3/16 inch) O.D. by 0.889 mm (0.035 inch) wall stainless steel tubing were tack welded to the center of the bottom pipe cap for monitoring external temperature. A thermowell, made from 3.97 mm (5/32 inch O.D.) by 0.889 mm (0.035 inch) wall stainless steel tube sealed at one end, penetrates through the center of the top pipe cap and into the titanium for monitoring internal temperature. Small thermocouples (TCs) were attached to the outside of the valve bodies to monitor their temperature. HSV-P2 had thermocouples attached to the "middle" and "top" locations indicated in Figure 2.
HSV-P1 and HSV-P2 had body in-leakage rates of 2´ 10-9 and 7´ 10-9 scc He/sec, respectively. The prototype vessels weighed approximately 20.4 kg (45 pounds) thus satisfying the 22.7 kg (50 pound) weight limit. The internal void volume of HSV-P1 was measured by gas expansion to be 3.997 L, while HSV-P2 was 3.780 L. Figure 3 is a radiograph of prototype vessel HSV-P1.

Figure 3. X-Ray of Prototype HSV-P1
A valve cooler, a device fabricated from aluminum to help maintain the HSV valves from exceeding their temperature rating, was installed for some tests. The valve cooler was used to support the HSV in a vertical furnace during desorption tests. The valve cooler was designed for use with a coolant flow through its body and air or nitrogen was used for some tests.
To ensure the HSV does not exceed it maximum design pressure rating at the maximum design temperature, the initial gas loading of the vessel must be limited. In the unlikely event the vessel was heated to its maximum design temperature with its valves closed or filters plugged, the maximum initial loading of the titanium can be calculated to limit the hydride desorption pressure to its design value.
A pressure versus Q/M correlation was developed for the d-phase region to determine Q/M corresponding to 760°C and 1425 kPa (10, 690 torr). A Log10(P) versus Q/M regression was conducted using the HY-STOR 106Ô deuterium isotherm data and the G&S Rod protium isotherm data in the d -phase region at 550, 600, 649, and 700° C. The equation relating P to Q/M for Q/M ³ 1.56 is
![]() 1
1
where the coefficient were determined by linear regressions to be b 0,0 = 23.86042, b 0,1 = -26255.4, b 1,0 = -8.09214, and b 1,1 = 11919.5.
A value of 1.620 Q/M at 760°C in Equation 1 gives a pressure of 1423 kPa (10,670 torr) which is slightly lower than the 1425 kPa (10, 690 torr) design pressure. A 4.0 L vessel void volume at 760°C and 1425 kPa (10, 690 torr) contains 14.9 STP-L of gas and is equivalent to 0.015 Q/M (for 4400 grams of titanium) if the gas is absorbed in the titanium at room temperature. The maximum initial loading to prevent over-pressurizing the vessel is the sum of these two values and is 1.635 Q/M.
Apparatus
A simplified schematic of the HSV test apparatus is shown in Figure 4. The manifold on the left side of the figure was constructed from of 6.35mm (1/4 inch) tubing, valves, and flex hoses while the right side of the manifold was constructed from of 12.7 mm (1/2 inch) tubing, and valves. The HSV was connected to gas manifold using 12.7 mm (1/2 inch) braided flex hoses. The calibrated volume was three, 50 liter, aluminum compressed gas cylinders. A pressure transducer, indicated in the figure as "PT", actually represents multiple pressure/vacuum instruments. On the left side of the manifold, PT represents a 1333 kPa (10,000 torr) transducer, a thermocouple vacuum gauge (TCVG), and for some tests, a cold cathode ion gauge. On the right side of the manifold, PT represents a 1333 kPa (10,000 torr) pressure transducer, a 133 Pa (1 torr) pressure transducer, and a cold cathode ion gauge. Various mass flow controllers (MFCs) were used to measure gas flow: those shaded in the figure were installed only for certain test configurations.
The gas for titanium absorption experiments could be supplied from the calibrated volume or directly from the lab gas supply. The gas was supplied to the pressure regulator, the flow controlled by the MFC, and fed to the bottom tube of the HSV by closing the manifold isolation valve which isolated the top HSV tube from the bottom HSV tube.
Activation
The goal of the hydride activation step is to have sufficiently active titanium so that at ambient temperatures and moderate hydrogen pressures, the titanium will start to absorb hydrogen and continue until it reaches its target loading without the addition of heat to the vessel.
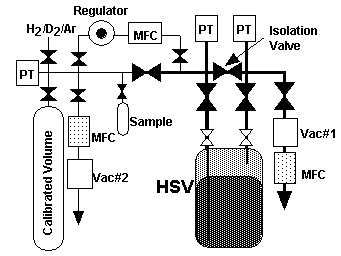
Figure 4. Test System Schematic
The HSVs were rotated slowly from the vertical to the horizontal position for activation so that the bottom HSV filter would still be covered with the titanium. The vessels were placed in a horizontal furnace resting on stainless steel straps to prevent contact with the furnace heating elements. Two activation methods were tried: 1) evacuation only while heating, and 2) argon purging while heating, followed by a heated evacuation. Vac#1 for activations was a Drytel 100 pump which consisted of a Alcatel 5030, a 27 liter per second (nitrogen) molecular drag pump, backed by its diaphragm pump.
HSV-P1 was activated by heating the vessel under vacuum and the temperature/vacuum history is shown in Figure 5. Three temperature traces shown in Figure 5 with the top profile being the furnace TC, the middle profile being for the HSV bottom TC, and the bottom profile being for the HSV internal TC. PUMP PT is the ion gauge reading for PT closest to the vacuum pump and BOTTOM PT is the ion gauge reading on the bottom tube of the HSV.
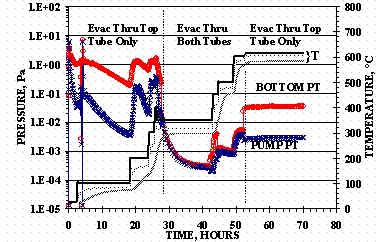
Figure 5. HSV-P1 Activation
The vessel was initially evacuated at ambient temperature. With the isolation valve open, the vessel was held at ambient for 3 hours, 100°C for 16 hours, 200°C for 6 hours, and 300°C for 2 hours. The decrease and increase in ion gauge readings at 3.6 hours was due to a ten minute closing the HSV bottom and top valves and opening of the isolation valve.
The isolation valve was then opened and the vessel held at 350°C for 17 hours, 450°C for 2 hours, 500°C for 5 hours, and 600°C for 3 hours. The final temperature of 615°C was held for 18 hours with the isolation valve closed. The final pressures of 8´ 10-2 Pa (6´ 10-4 torr) for the bottom tube side and 8´ 10-3 Pa (6´ 10-5 torr) for the top tube side were virtually constant during the entire 615°C bake-out time. The total activation time was 70 hours with an additional 18 hours required for the vessel to cool-down while its was still inside the furnace.
The pressure and temperature data for HSV-P2 activation is shown in Figure 6. The HSV was activated by purging with 0.5 SLPM argon, greater than 99.99% purity, for 3 hours with the furnace turned on to a set point of 350°C at the start of the purge. The flow, fed to into the bottom tube and out the top tube (isolation valve closed), gave inlet pressures between 12.7 to 13.9 kPa (95 and 104 torr) at the bottom tube and 2.93 kPa (22 torr) at the top tube. After stopping the argon purge, the isolation valve was opened and HSV evacuated at 350°C for 5 hours, at 450° C 2 hours, at 550°C for 1 hour, and at 615°C for 12 hours.
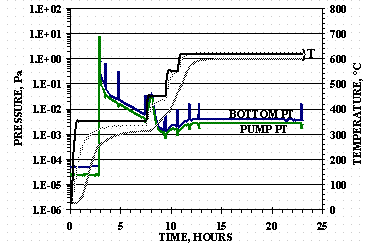
Figure 6. HSV-P2 Activation
Vacuum levels of the material were monitored periodically during the activation by closing the isolation valve, waiting for the ion gauge readings to stabilize, and then reopening the isolation valve. These vacuum level checks can be seen in Figure 6 as pressure spikes and dips. The three temperature traces shown in Figure 6 show at the end of the activation, the top profile is the HSV bottom TC, the middle trace the furnace TC, and the bottom TC profile the HSV internal TC. The difference in order for the furnace TC and the bottom TC for HSV-P1 and HSV-P2 is most likely due to variations in the placement of the HSVs in the furnace.
The HSV-P2 final activation pressures of 4´ 10-2 Pa (3´ 10-4 torr) at the bottom tube and 4´ 10-3 Pa (3´ 10-5 torr) for the top tube, with the isolation valve closed, are within instrument error of the values obtained for HSV-P1. These final values were virtually constant during the entire 615° C bake-out. The total activation time was 23 hours with only an additional 5.4 hours of cooling required since the furnace was opened at the end of the bake-out and the vessel removed from the furnace after 2.8 hours of cooling.
HSV Loading/Hydriding
After activation and cooling of the HSVs, the vessels were allowed to sit idle for five days to simulate the anticipated delay between activation and loading tritium into production vessels and to test the quality of the activation process. The HSV was place vertically on rack in a ventilated hood for absorption/loading tests. Deuterium flushes of the manifold piping were done before the first absorption test for both HSVs to minimize the gas impurities which could poison the titanium. All absorption/loadings were done through the bottom tube of the HSV unless stated otherwise.
Some HSV loadings where conducted by filling calibrated volumes with hydrogen or deuterium and controlling the supply pressure to the MFC with a pressure regulator. Most absorption tests were run by supplying gas directly from the laboratory supply to the pressure regulator. HSV gas loadings were calculated by integrating MFC flow-time data and converted to X/M or Q/M values: pressure-volume-temperature (PVT) measurements for tests using the calibrated volume gave gas quantities within ![]() 3 percent of the values calculated using integrated flow data. Q/M gas inventories at the end of HSV-P1 desorption tests were estimated using the vessel’s steady-state desorption pressure, temperature, and the bench scale isotherm data. Q/M desorption inventories for HSV-P2 were calculated using integrated vacuum pump discharge MFC flow-time data.
3 percent of the values calculated using integrated flow data. Q/M gas inventories at the end of HSV-P1 desorption tests were estimated using the vessel’s steady-state desorption pressure, temperature, and the bench scale isotherm data. Q/M desorption inventories for HSV-P2 were calculated using integrated vacuum pump discharge MFC flow-time data.
Results
HSV-P1 Absorption Tests
For the initial (Cycle 1) absorption test, the calibrated volume was filled with deuterium and fed to the HSV through the MFC at 10 SLPM. Hydriding started immediately and continued at 10 SLPM until the loading was stopped to refill the calibrated volume and again at the end of the test. A total of 1845 STP-L of deuterium were loaded in 193 minutes. An additional 93 STP-L of deuterium were added the following day to bring the total Cycle 1 loading to 1938 STP-L (1.883 D/M).
After the Cycle 1 absorption test, an isotopic exchange test at 600°C was attempted for HSV-P1 using a protium flow of 2.5 SLPM. The test was interrupted after 147 minutes due to an insufficient protium supply and was continued the next day for another 1075 minutes. The HSV-P1 inventory was an estimated 1.690 Q/M (1739 STP-L) at the end of the exchange test using titanium isotherm data. HSV-P1 inventory after the first desorption cycle was estimated to be 0.285 Q/M (293 STP-L)
Before the second absorption cycle (Cycle 2), air was introduced to the space between the flex line valves and the HSV valves, and the estimated 11.2 cc of air deliberately admitted into the HSV. This was done to test the sensitivity of the titanium to air exposure after the material had already been through one hydride cycle. Then, the Cycle 2 absorption test at 15 SLPM was performed using protium supplied directly to the pressure regulator: the final gas inventory was 1.757 Q/M (1809 STP-L).
Cycle 2 hydriding of HSV-P1 started immediately and was constant until 87 minutes into the test when the vessel pressure increased to the regulator supply pressure of approximately 180 kPa (1350 torr) and the internal TC temperature of 655°C was reached. The supply pressure was decreased and hydriding continued slowly as the vessel cooled. The 1515 STP-L (1.472 H/M) of gas added to the bed was completed in 121 minutes.
The third absorption cycle (Cycle 3) at 7.5 SLPM, after Cycle 2 desorption to 0.170 Q/M, was performed using protium supplied directly to the pressure regulator and gave a final inventory of 1.960 Q/M (2018 STP-L). Cycle 3 hydriding started immediately and was constant for 229 minutes until the vessel's pressure increased to the regulator supply pressure of approximately 139 kPa (1043 torr). The supply pressure was maintained at this pressure and hydriding continued at a slower rate as the vessel cooled. The internal HSV temperature was relatively constant at 490 ± 10°C for over two hours and increased to its maximum value of 527°C after 203 minutes, after which it decreased as the pressure increased. The 1843 STP-L (1.790 H/M) of gas added to the bed was completed in 280 minutes
HSV-P1 gas inventory history for the absorption and desorption tests is shown in Figure 7. Figure 8 shows the HSV inlet and outlet tube absorption pressures versus Q/M for Cycles 1, 2, and 3: the outlet tube valve for Cycle 1 absorption was closed and not plotted in the figure. Figure 9 shows HSV-P1 absorption temperatures for the internal TCs and the bottom TCs versus Q/M for Cycles 1, 2, and 3.
Figure 8 and Figure 9 show how loading pressure and temperature affect the HSV gas absorption rate. The absorption supply pressure at a gas feed of 7.5 SLPM, Cycle 3, was the lowest of the three tests and did not generate a significant pressure at the HSV top tube until a loading of 1.5 Q/M had been reached. After 1.5 Q/M, the inlet and outlet tube pressures approach one another and when within a circa 6.67 kPa (50 torr) differential, the gas flow rate dropped below the 7.5 SLPM set point value: the HSV temperatures had already reached their maximums values before the flow rate started to decrease.
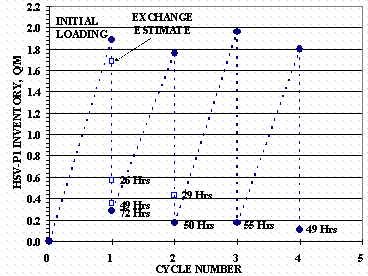
Figure 7. HSV-P1 Cycle History
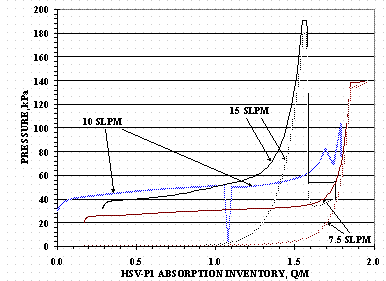
Figure 8. HSV-P1 Absorption Pressures
The HSV-P1 absorption pressure at 10 SLPM was roughly equal to that at 15 SLPM for a relative increase in inventory from 0 to 0.7 Q/M (720 STP-L). After an increase of 0.7 Q/M, the pressure required to maintain the 15 SLPM absorption rate was greater than that needed to maintain the 10 SLPM absorption rate. When the HSV top tube pressure approached within circa 13.3 kPa (100 torr) of the inlet pressure during the 15 SLPM loading test, the flow rate drops below 15 SLPM: the HSV temperatures reached their maximums shortly after the flow rate decreased.
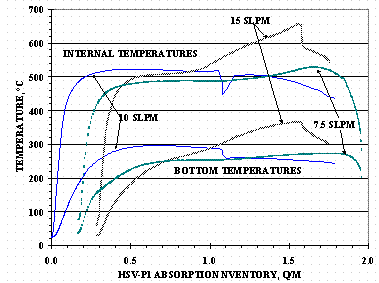
Figure 9. HSV-P1 Absorption Temperatures
Use of a 1500 L tank at 147 kPa (1100 torr) had been proposed for loading HSVs and it was questioned if an HSV could exceed its 760°C design temperature if it was loaded directly from the tank instead of through a flow control device. The absorption tests demonstrated temperatures greater than 655°C could be reached if the titanium absorption gas pressure was high enough and has a sufficient gas supply to sustain the hydriding reaction.
HSV-P1 Cycle 4 absorption, after Cycle 3 desorption to 0.170 Q/M (175 STP-L), was performed to simulate the direct loading of an HSV from a 1500 L tank starting at 147 kPa (1100 torr). For this test, the calibrated volume was filled with protium and the pressure regulator set to approximately 147 kPa (1100 torr). Flow was initiated to the HSV and allowed to continue until approximately 170 STP-L of gas were loaded into the vessel. The loading was stopped momentarily, the pressure regulator adjusted down to the approximate pressure expected for an equivalent gas transfer from a 1500 L tank, and the loading continued. These steps were repeated for equivalent 1500 L tank pressures down to 66.7 kPa (500 torr) at which time the calibrated volumes were refilled with protium. The test continued until a total of 1672 STP-L (1.624 H/M) had been added to the bed bringing the total loading to 1847 STP-L (1.794 Q/M) in 132 minutes. Figure 10 is a plot of some of the data for HSV-P2 Cycle 4 absorption.
The initial protium flow rate is over 90 SLPM and in the first 20 minutes drops down successively to flows in the range of 84, 70, 53, 36, and 20 SLPM for each adjustment of the regulator pressure. An exponential function was fitted to the median flow rate values in their time interval for the first 20 minutes and is shown in Figure 10 to give an approximate representation of unrestricted hydriding flow as a function of time at the conditions tested. The maximum internal HSV temperature obtained was 653°C which is lower than the vessel design temperature of 760°C.
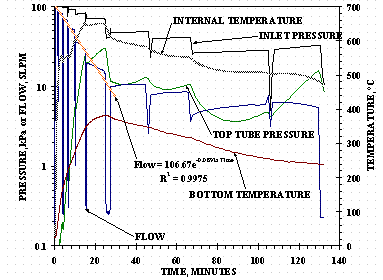
Figure 10. HSV-P1 Simulated 1500 L Tank Loading
HSV-P1 Desorption Tests
For HSV-P1 desorption Cycle 1, a 100 SLPM MFC was installed between the manifold and Vac#2 – an Edwards ESDP 30 scroll pump. The vessel was already heated to 600°C from the exchange test when the desorption was started using Vac#2 with the isolation valve closed. HSV-P1 was evacuated for 18 hours with an estimated 1065 STP-L of gas removed from the vessel. The furnace set point was then increased to 625°C and the MFC used for another 4 hours before being removed giving an estimated gas removal of 1175 STP-L. During MFC removal, Vac#1 – a Drytel 100 vacuum pump system, was used to evacuate the vessel. After MFC removal, Vac#2 and Vac#1 were both used for vessel desorption.
Desorption pressures were measured periodically during the tests by momentarily valving-out the vacuum pumps and recording the HSV gas pressure. These pressures were used with the isotherm data to estimate the residual gas on the HSV. HSV-P1 was evacuated for a total of 72 hours for Cycle 1. Isotherm estimates of HSV-P1 inventories were 0.570 Q/M at 26 hours, 0.355 Q/M at 49 hours, and a final inventory estimate of 0.285 Q/M. These values are shown in Figure 7.
For HSV-P1 desorption Cycle 2, a 5 SLPM MFC was installed between the manifold and the Vac#2 Edwards scroll pump. HSV-P1 desorption started with the vessel at ambient temperature, the isolation valve open, and a furnace set point step change to 600°C. After 16 hours, the furnace set point was changed to 650°C and then to 700°C eight hours later. At 29 hours into the test, the Vac#1 Drytel 100 pump was used while the MFC was removed from Vac#2 line: Vac#1 and Vac#2 were both used for the remainder of the desorption.
The maximum evacuation flow recorded was 4.8 SLPM, but flows above 1 SLPM were not measured after 6 hours of evacuation. From MFC data, it was estimated that 1364 STP-L of gas were desorbed before the MFC was removed. At the end of the 50 hour desorption, the gas inventory was estimated to be 0.170 Q/M.
For HSV-P1 desorption Cycle 3, the Vac#2 Edwards pump was used with no MFC installed. Vac#1 used only the diaphragm pump of the Drytel 100 vacuum pump. The desorption started with the vessel at ambient temperature, the isolation valve open, and a furnace set point step change to 700°C. After 22 hours of evacuation, HSV-P1 valves were closed, while the vessel was still being heated, and the bottom tube connected by a 6.35 mm (1/4 inch) flex line to separate manifold using a Drytel 30 vacuum pump: a 7.5 liter per second (nitrogen) molecular drag pump backed by its own diaphragm pump. HSV-P1 was evacuated for another 34 hours at 700°C and gave a final gas inventory estimate of 0.170 Q/M – the same as the Cycle 2 desorption.
For HSV-P1 desorption Cycle 4, the Vac#2 Edwards pump was used, with no MFC installed in the vacuum line, for the first 5 hours of the evacuation: the remainder of the evacuation was done solely by the Vac#1 Drytel 100 pump. The desorption started with the vessel at ambient temperature, the isolation valve open, and a furnace set point step changed to 700°C. The vessel was evacuated for a total of 49 hours and gave a final gas inventory estimate of 0.110 Q/M.
HSV-P2 Absorption and Desorption Tests
As was done for HSV-P1, HSV-P2 was allowed to sit idle for five days before the initial hydriding. For the Cycle 1 absorption test, the calibrated volume was filled with deuterium and fed to the HSV through the pressure regulator and MFC at 5 SLPM. Hydriding started immediately and was constant at 5 SLPM until the loading was stopped to refill the calibrated volume with deuterium. HSV-P2 absorbed deuterium at 5 SLPM for a total of 264 minutes before the absorbed rate decreased and the deuterium pressure over the titanium approached the regulator set point of 140 kPa (1050 torr). Loading continued at a reduced rate for another 89 minutes after which the loading was stopped: the rate was 0.8 SLPM. A total of 1501 STP-L of deuterium (1.434 D/M) were loaded onto the vessel.
It was decided to load more deuterium into HSV-P2. To determine what temperature was needed to restart the hydriding, the vessel was suspended vertically in a furnace. Starting from ambient temperature with a furnace set point change to 300°C, hydriding started 53 minutes later when the bottom temperature reached 165°C and the internal temperature reached 104°C. The hydriding rate increased to a maximum of 4.5 SLPM and then slowly decreased. The loading was stopped after 166 minutes: the final Cycle 1 inventory was 1802 STP-L (1.722 D/M). This test demonstrated that insufficiently loaded or deactivated material can be hydrided at moderately low temperatures to finish the HSV loading.
After the initial loading of HSV-P2, the vessel sat dormant for almost 40 months before cycling tests continued. The uncertainty of estimating HSV-P1 desorption inventories using isotherm data lead to test manifold modifications to better measure gas desorption. Vac#1 consisted of an Alcatel 5030 molecular drag pump backed by an Edwards ESDP 30 scroll pump. A filter was installed on the exhaust of the scroll pump, to dampen out pressure fluctuations of the pump, and was connected to two MFCs, a 5 SLPM and a 2 SLPM installed in parallel, to measure the exhaust gas flow rate. Tests flowing protium through the manifold regulator and controlled by the manifold MFC at 6 SLPM gave a manifold pressure of circa 3.20 kPa (24 torr) and a total exhaust flow of 6 SLPM: the 2 SLPM MFC was at its full scale flow and the 5 SLPM MFC at 80% of its range.
HSV-P2 cycling tests were conducted to determine the effectiveness of the isotopic exchange process, but only absorption/desorption results will be presented. At the start of a desorption test, the furnace set point was changed to 700°C and desorption performed using Vac#1 for a total of 24 hours. When the desorption gas flow dropped below 2 SLPM, the 5 SLPM MFC was isolated to obtain better gas flow measurements. The amount of gas removed from HSV-P2 was calculated by integrating the flow-time data. The amount of gas to be added during the next absorption cycle was calculated to obtain a target absorption loading of 1.55 Q/M.
The first desorption cycle was done in two parts: the first part was to get the initial HSV inventory from 1.722 D/M closer to the target 1.55 D/M and the second part to perform a 24 hour desorption. The remaining cycles were done as described. Figure 11 shows the cycling inventory history for HSV-P2.
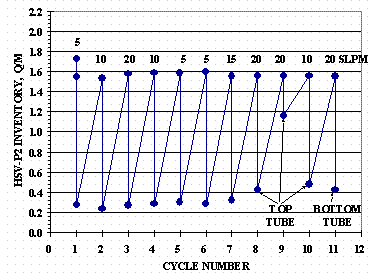
Figure 11. HSV-P2 Cycle History
The absorption rates for each cycle are shown in Figure 11. All absorptions were through the bottom HSV tube except Cycle 11 which was fed through the top tube by switching the order of the manifold flex lines to the HSV. Desorption for Cycles 1 through 7 were done with the isolation valve open. Desorption Cycles 8, 9, and 10 were done with the isolation valve closed thus evacuating gas only through the top HSV tube. Excessive scroll pump exhaust pressure, due to particles shed by the pump accumulating on the filter, interrupted the Cycle 9 desorption test. Cycle 11 desorption was through the bottom HSV tube only and was done by switching the order of the manifold flex lines to the HSV.
The absorption pressures and temperatures for some of the HSV-P2 loadings using the bottom tube at various flow rates are shown in Figure 12 and Figure 13. The 20 SLPM loading in Figures 12 and 13 was stopped short of the target loading due to an insufficient protium supply and the loading to the value shown in Figure 11 completed after replenishing the gas supply.
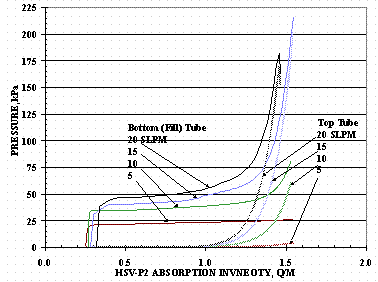
Figure 12. HSV-P2 Absorption Pressures
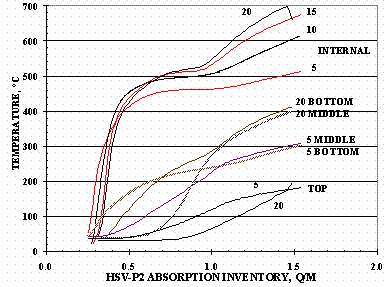
Figure 13. HSV-P2 Absorption Temperatures
Figure 14 shows the absorption pressures and temperatures for HSV-P2 when loading from the top HSV tube. It is interesting to note that at 20 SLPM, the fill tube absorption pressure is lower for top loading an HSV than the fill tube pressure for bottom tube loading. Also, the internal HSV temperature is higher for top loading than for bottom loading – even with the top loading starting at a higher Q/M value.
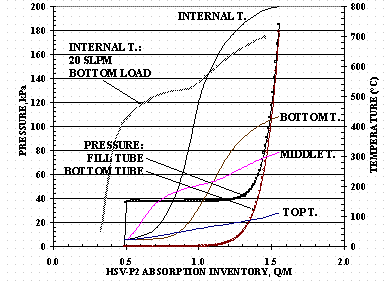
Figure 14. HSV-P2 20 SLPM Top Loading
Discussion
The two different activation techniques showed activation is enhanced when a heated inert/dry gas purge at moderate temperature is used before vacuum evacuation using both vessel tubes. The activation technique showed that the titanium remains activate even five days after activation.
HSV loading rates of 15 and 20 SLPM generate internal HSV temperatures over 600°C. Absorption rates at 10, 7.5, and 5 SLPM take longer to reach target loadings, but allow time for the hydriding heat of reaction to be dissipated by various heat transfer mechanisms. The hydriding heat of reaction can be used somewhat to over-come deactivation of the titanium by heating the titanium to a sufficient temperature to keep the titanium hydriding and activating at the same time. HSV loading directly from a 1500 L tank at 147 kPa (1100 torr) will not cause the HSV to exceed its design temperature, but use of a flow control device is recommended. Loading at lower pressures is an option, but reduces the driving force for absorption. Loading multiple times from a smaller tank can also be used to limit the temperature rise of the titanium during absorption.3
Some titanium deactivation was present in HSV-P2 which prevented the 5 SLPM loading of the HSV above 1.28 D/M. The heating of HSV-P2 to complete its loading was minimal and could have been supplied by the hydriding reaction if the vessel was loaded at a faster rate. Loading HSV-P2 at 7.5 to 10 SLPM would most likely have heated the titanium to a temperature that would have allowed complete loading of the vessel without external heating.
Titanium isotherm data show that above approximately 1.5 Q/M, the titanium d-phase is present and higher pressures are needed to load in this hydride phase. The maximum initial hydride loading to protect the vessel’s pressure boundary was shown to be 1.64 Q/M (1688 STP-L). To avoid approaching this loading limit, a nominal target of 1.55 Q/M (1595 or a nominal 1600 STP-L for a 4400 gram titanium bed) is recommended. This is slightly into the titanium d-phase, but allows for a nominal buffer of 93 STP-L of gas to be loaded into the vessel before reaching the upper loading limit.
HSV desorptions at 700°C gave lower titanium gas inventories that using lower temerpatures. HSV-P1 desorptions were hindered by the initial use of the Edwards scroll pump and the placement of the MFC between the HSV and the vacuum pump. HSV-P2 desorptions showed an Alcatel 5030 molecular drag pump could be used for the entire desorption time without over loading the pump with a furnace ramp from ambient to 700°C in 2.5 hours.
Desorption through both HSV-P2 tubes removed an average of 1346 STP-L in 24 hours while desorbing through the top tube only averaged 1158 STP-L in 24 hours: a difference of 188 STP-L. HSV-P1 desorptions for longer than 24 hours showed lower titanium gas inventories could be obtained, but the qualifying these levels was difficult due to the quality of the isotherm data at high temperatures and low pressures.
A nominal tritium disposal limit of 3.70x1013 Bq (1000 Curies) for an HSV would give a 0.00038 T/M inventory. Desorption levels are not near this level and isotopic exchange will be needed to meet waste acceptance criteria for disposal. An enlarged filter surface area would likely aid in desorption of gas from the HSV and may reduce the initial tritium inventory in the vessel before the start of isotopic exchange and reduce the number of cycles to be performed.
Titanium has the ability to retain He-3 in its metal hydride to relatively large He-3/M ratios. Using titanium films loaded with tritium9, He-3 was shown to be retained by titanium above 0.3 He-3/M. Figure 15 shows the time before the start of He-3 release from titanium as a function of He-3/M capacity and initial grams of tritium in 4400 grams of titanium -- a theoretical maximum of 554 grams of tritium. The times in Figure 15 do not include the additional time for He-3 to pressurize the HSV’s pressure vessel which can add several years to a vessel’s storage life. Selecting the desired storage life for the vessel, i.e. the amount of time before tritium recovery is to begin, will indicate how much tritium can be stored in each HSV.
Conclusions
Titanium in an HSV gives a higher tritium capacity by weight than many other hydrides and the advantage of significant He-3 retention before release from the material. Titanium, manufactured by a commercial process as Ergenics HY-STOR 106Ô , has been shown through cold testing to be a suitable material form for storing tritium. 4400 g of HY-STOR 106Ô in an HSV can be activated, loaded at ambient temperature and moderate flow rates without exceeding vessel design temperatures, and desorbed to moderately low inventories at 700°C. The desired storage life for the tritium in the HSV will dictate how much tritium can be stored by an HSV.
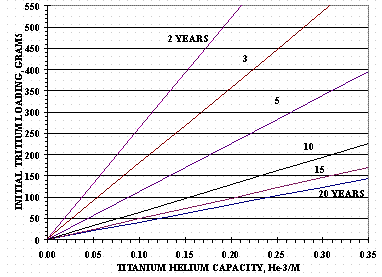
Figure 15. He-3 Release Times for 4.4 kg Ti
Acknowledgments
The author would like to thank Kit Heung and Joe Wermer for their contributions to this work. This document was prepared in connection with work done under Contract No. DE-AC09-96SR18500 with the U.S. Department of Energy.
References