WSRC-MS-2001-00155
Rapid Mass Spectrometry Method for
Uranium and Plutonium
S. L. Maxwell, III and J. Satkowski
Westinghouse Savannah River Company
Aiken, SC 29808
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Abstract
A new, rapid sample preparation method to improve the assay of uranium and plutonium by mass spectrometry has been developed at the Westinghouse Savannah River Site (SRS). The method is applied to the analysis of mixed plutonium-uranium oxide materials but can be applied to low-level mass spectrometry analyses as well. The new method uses a single extraction chromatographic column to separate and purify both uranium and plutonium for isotopic assay by thermal ionization mass spectrometry. A dual uranium/plutonium spike isotope dilution technique is used to allow rapid analysis of uranium/plutonium content and isotopics. A single UTEVA Resin column is utilized to simultaneously separate both uranium and plutonium from the sample matrix. The plutonium and uranium can rapidly removed from the column separately or eluted together for analysis. This new method combines sample preparation into a single, effective separation step that significantly reduces sample preparation time and costs.
Introduction
Thermal ionization mass spectrometry (TIMS) is often used to perform uranium and plutonium isotopic measurements on a variety of sample types. It can be utilized to provide analytical support for nuclear material processing as well as for the low-level analysis of plutonium and uranium in urine (1). New extraction chromatographic resins from Eichrom Technologies, Inc. coated with highly selective extractants have been used to improve actinide separation techniques for laboratory use (2, 3). At the Savannah River Site Central Laboratory, plutonium is typically separated and purified for mass spectrometry isotopic analysis using TEVA Resin and uranium is separated using UTEVA Resin (4). This technology is a significant improvement over solvent extraction methods that were used previously that generated mixed waste and were much less efficient.
Mixed uranium-plutonium oxide materials characterized in the SRS Central Laboratory are analyzed for plutonium/uranium content and isotopic abundance using thermal ionization mass spectrometry (TIMS). In the previous SRS method, uranium and plutonium isotopic analyses were processed separately. The previous method required two separate column methods (TEVA and UTEVA resins) and separate instrument analysis methods. Each method required four to six hours of sample preparation time that includes sample dilution, addition of sample to U-233 or Pu-244 spike, column extraction separations, filament plating, instrument analysis, and calculation of final results.
A new UTEVA single column separation was recently developed to separate and purify plutonium and uranium on a single UTEVA Resin column to reduce the time required to prepare the samples for analysis by TIMS. This method was tested and compared to the current established methods at SRS. The new UTEVA method has two analysis options: single filament and two filament. In the new method, plutonium and uranium can be collected separately (single UTEVA column separation with two filament mounting) or together (single UTEVA column separation with single filament mounting). The single filament method can be used when uranium-plutonium ratios are within an acceptable range so that U and Pu can be analyzed simultaneously by the mass spectrometer. Since Pu-238 is typically analyzed in the SRS Lab by alpha spectrometry (due to its low isotopic abundance and potential interference from U-238), U-238 does not interfere with Pu-238 in this method when uranium and plutonium are purified and collected together.
Experimental
Reagents
The resins employed in this work are TEVA Resin (AliquatÔ 336) and UTEVA-Resinâ (diamylamlyphosphonate) available from Eichrom Technologies, Inc., Darien, Illinois. Nitric and hydrofluoric acids were prepared from high -purity OptimaÔ reagents (Fisher Scientific, Inc.). All water was obtained from a Milli-Q2 water purification system. All other materials were ACS reagent grade and were used as received. The isotope spikes Pu-244 (0.70 ug ) and U-233 (140 ug) were obtained from Oak Ridge National Laboratory and was validated by the SRS Quality Control Group using SRM 949 plutonium metal from New Brunswick Laboratory (Chicago, IL) and SRM 960 uranium metal from National Institute of standards and Technology (Gaithersburg, MA).
Procedures
Sample Digestion. Mixed oxide samples (0.5 gram aliquots) were digested in 10 milliliters of 12M nitric acid-0.1M hydrofluoric acid at 220°C for approximately 30 minutes using microwave heating. Each sample was diluted to 25 milliliters using 4 M nitric acid. An additional 1 to 10 dilution by weight was performed to lower the uranium/plutonium levels for transfer of material from glovebox to a hood containment.
Column preparation. The U-TEVA columns were prepared using 2 mL of resin. Chromatographic-UTEVA-resin columns were prepared by slurrying the appropriate resin in water, then transferring aliquots of the slurry under vacuum to a column body (Image Molding, Commerce City, CO) until the desired bed height was reached. TRU resin and SR Resin pre-packed cartridges containing 2 mL of resin were obtained from Eichrom Technologies. Small particle size (50-100 micron) was employed, along with a Speedmate-16 vacuum extraction system (Applied Separations, Inc., Allentown, PA, or Eichrom Technologies). Flow rates of 1 -2 mL/min were typically used, much faster than the 0.25 mL/min gravity flow rates typically observed.
Sample Preparation and Testing. A batch of five mixed oxide samples were prepared for TIMS analysis. The weight percent uranium ranged from 25.0 % to 58.6% and the plutonium content ranged from 7.3% to 22.2% in the mixed oxide samples. For each sample mixture, uranium in each sample mixture was purified using a separate UTEVA column method and plutonium was purified using a TEVA method described previously (5). The digested, diluted sample solutions prepared in the glovebox were diluted again by weight using a 1 to 10 dilution. Corresponding sample aliquots were added to separate U-233 and Pu-244 spike materials and the separate column extractions were performed. A small volume of each purified uranium and plutonium solution was plated on separate filaments and subsequently analyzed by TIMS. Isotopic abundance and concentration calculations were performed.
For comparison, the same five samples were also prepared again using a 1 to 5 dilution and the single UTEVA column, two-filament scheme. Aliquots of each sample dilution were added to dual spike material containing both U-233 and Pu-244. These dual spikes were previously prepared to contain both uranium and plutonium spike materials having an amount equivalent to the separate spike preparations. The mixture was purified using the new single column (UTEVA) method, while separating the uranium and plutonium fractions for subsequent analysis on two separate filaments using TIMS (Figure 1). A quality control standard was also analyzed using this method.
The sample aliquot and mixed U/Pu spike aliquot were weighed and 4 milliliters of 2.5 M nitric aicd-0.5M aluminum nitrate (previously scrubbed with UTEVA resin to remove traces of uranium) were added. Two hundred microliters of 1.5 M ferrous sulfate was added to reduce plutonium to Pu (III), followed by 400 microliters of 3M sodium nitrite to oxidize the plutonium to Pu (IV). After conditioning the column containing one milliliter (mL) of UTEVA resin with 3 mL of 3 M nitric acid, the sample-spike solution was added to each column. Each vial was rinsed with 3 mL of 3 M nitric acid and this rinse was added to the appropriate column. Each column was rinsed with two 5 mL volumes of 3 M nitric acid. To elute plutonium separately (two filament method), 5 mL of 3M nitric acid-0.2M hydrofluoric acid was added to the columns. Uranium was then eluted using 5 mL of 0.02M nitric acid-0.005M hydrofluoric acid. The strip solutions containing plutonium with the 3M nitric acid-0.2M fluoride were ashed three times to dryness to ensure fluoride was adequately volatilized, adding 3M nitric acid each time to rinse vial walls.
Isotopic abundance and concentration calculations were performed. The Pu 238/240 ratio obtained from alpha-particle pulse height analysis results was used to calculate Pu-238 due to its low abundance, as was used in the two column (TEVA, UTEVA) method described previously.
Five of the same samples previously diluted 1 to 5 were again selected for single UTEVA column, single filament testing. Single aliquots of each sample were mixed with the dual spike materials. The purification was performed using a single UTEVA column, but uranium and plutonium were eluted from the column together in the same faction. To elute uranium and plutonium together, the 3 M nitric acid-0.2M hydrofluoric acid elution step described above was not used. Instead, uranium and plutonium were eluted together by adding 5 mL of 0.02M nitric acid-0.005M hydrofluoric acid to the UTEVA columns. Both the uranium and the plutonium measurement data were obtained from the same filament. The Pu 238/240 ratio obtained from alpha-particle spectrometry results was used for calculations to determine Pu-238, similar to the two column (TEVA, UTEVA) procedure. A quality control standard was also analyzed using this method.
Apparatus
A Finnigan MAT 261 thermal ionization mass spectrometer was used to perform the measurements. Microwave digestion of the oxide samples was performed using a Questron Q-Wave 3000 closed-vessel system with temperature and pressure monitoring. Microwave vessels that can handle up to 625 psi pressure were employed. Plutonium-238 measurements were performed by alpha-particle pulse-height measurements using surface- barrier-silicon detectors.
Results and Discussion
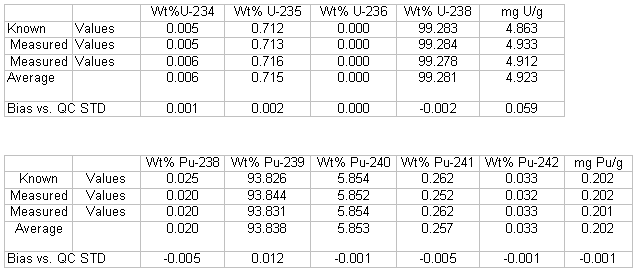
Table 1 shows uranium and plutonium isotopic as well as uranium and plutonium concentration measured values on a quality control standard using the UTEVA-single filament method. The bias results versus the known values are similar to what is typically observed using the current method. Table 2 shows uranium and plutonium isotopic as well as uranium and plutonium concentration measured values on a quality control standard using the UTEVA-two filament method. The bias results versus the known values are again similar to what is typically observed using the current method. The replicate precision observed is also acceptable to meet analytical needs in the SRS Laboratory.
The mass spectrometry results on the five mixed oxide samples were compared to determine if results using the new methods were statistically different from the current method. The differences in individual uranium and plutonium results were tested for statistical significance using a paired t-Test. Table 3 shows differences obtained using the previous method (TEVA /U-TEVA, 2 filament) and the UTEVA column-2 filament method. Table 2 shows differences obtained when comparing isotopic and concentration results using the previous method versus the UTEVA- 1 filament method. The critical t-test value for 4 degrees of freedom at the 99% confidence level is 4.60.
Uranium isotopic results in Table 3 indicate no significant difference due to the sample preparation method for all isotopes. The paired t-test values are all less than 4.60. Uranium isotopic results in Table 4 indicate there may be a statistically significant difference for U-238 since the paired t-test result of 5.68 is greater than 4.60. Even though there may be a slight statistically significant difference, the difference is very small from a practical standpoint and indicates adequate agreement between the two sample preparation methods for SRS laboratory purposes. The small test size may also not have given a true estimate of the standard deviation, affecting the t-test result. For example, a larger standard deviation for U-238 was seen in Table 3.
The results for the uranium concentration assay in Tables 3 and 4 indicate that the preparation scheme did not significantly affect the relative results for each sample versus the current method.
The plutonium isotopic values in Table 3 indicate no significant difference veersus the current method due to the sample preparation method for all isotopes. The paired t-test values are all less than 4.60. The Pu238 isotope was not measured during the mass spectrometry instrument analysis. The Pu238/240 ratio was obtained from previous alpha-particle spectrometry data and applied to the results.
Plutonium isotopic results in Table 4 indicate there may be a statistically significant difference for Pu-241 and Pu-242 since the paired t-test results of 9.38 and 4.68 respectively are greater than 4.60. Even this may be a statistically significant difference, the difference is very small from a practical standpoint and indicates adequate agreement between the two sample preparation methods for SRS laboratory purposes. The small test size may also not have given a true estimate of the standard deviation (smaller than what was seen in Table 3 for Pu-241, for example, affecting the t-test result. For two key Pu isotopes, Pu-239 and Pu-240, the t-test results in Table 4 were slightly less than the values shown in Table 3.
Results in Tables 3 and 4 indicate that the total plutonium assay results using the single filament and two filament method were not significantly different versus the current mass spectrometry method.
Major isotopes of interest in the SRS Laboratory are shown in Figures 2 and 3. Figure 2 plots the data for U-235, U-238, Pu-239, Pu-240 as well as total uranium and plutonium for the current method versus the UTEVA-single filament method. Figure 3 plots the data for U-235, U-238, Pu-239, Pu-240 and total uranium and plutonium for the current method versus the UTEVA-two filament method. The small differences observed versus the current mass spectrometry method further illustrate that the new method options can be used successfully.
Conclusions
The new method options using a single UTEVA extraction method was successfully tested and implemented for mixed uranium and plutonium oxide samples. This method will enable faster analysis times and significant labor cost savings due to decreased sample preparation time. When uranium and plutonium ratios fall with appropriate ranges, the UTEVA-single filament method can be used. The two filament option in which uranium and plutonium are separated from each other using UTEVA resin and mounted onto different filaments can also be used as needed.
Acknowledgment
This work was performed under the auspices of the Department of Energy, DOE Contract No. DE-AC09-96SR18500. The authors wish to acknowledge Shermette Upton for her assistance in testing this method. The authors also wish to acknowledge A. Harper Shull, statistician at the Westinghouse Savannah River Co., for his statistical evaluation of the data.
References
Table 1. Quality Control Standard Analysis By New Method (UTEVA - 1 Filament)

Table 2. Quality Control Standard Analysis By New Method (UTEVA - 2 Filament)
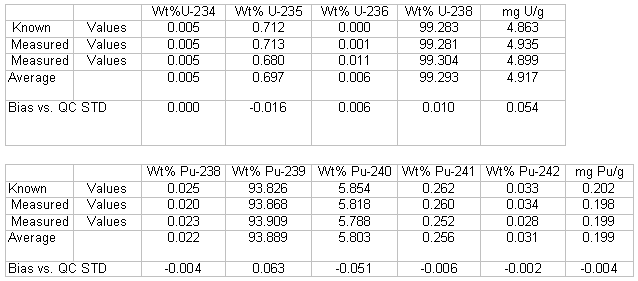
Table 3. Current Method vs. New Method (UTEVA - 1 Filament)
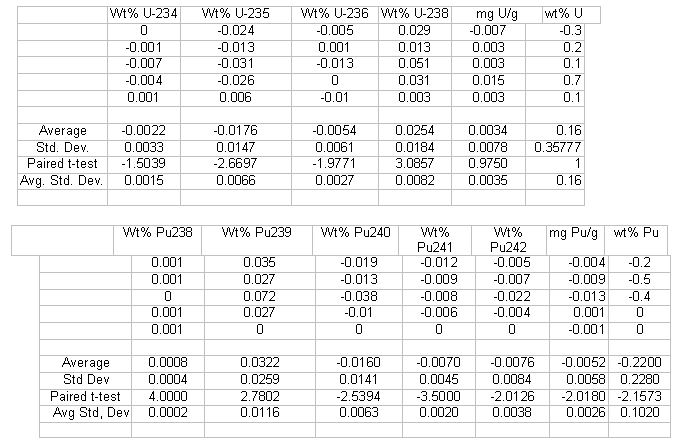
Table 4. Current Method vs. New Method (UTEVA - 2 Filament)
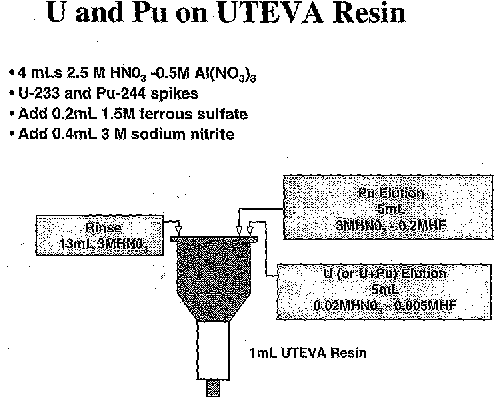
Figure 1. U-TEVA Column Separation
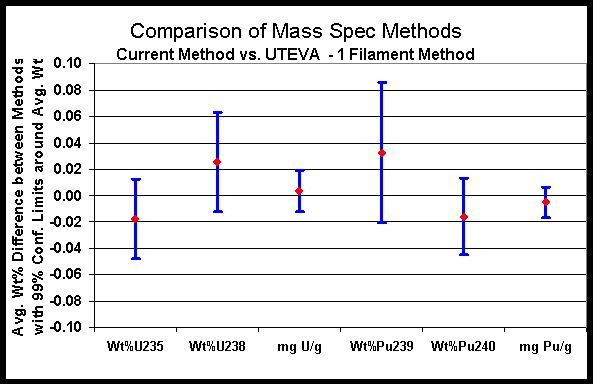
Figure 2. Comparison of Mass Spectrometry Methods (UTEVA - 1 Filament)
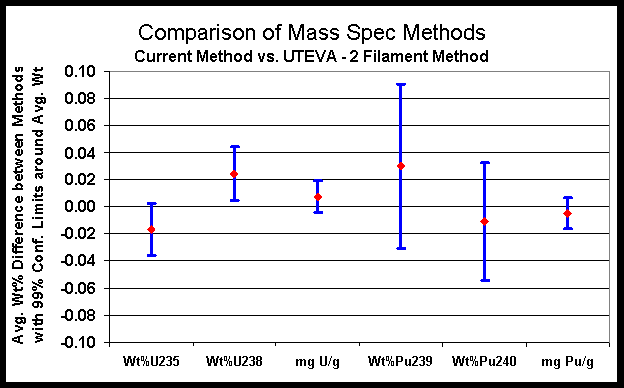
Figure 3. Comparison of Mass Spectrometry Methods (UTEVA - 2 Filament)