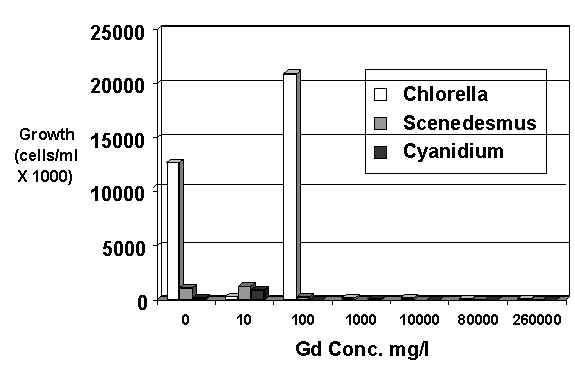
Figure 1. Growth of three species of algae at various concentrations of gadolinium
WSRC-MS-2000-00638
Toxicity of Gadolinium to Some Aquatic Microbes
E. W. Wilde and C. J. Berry
Westinghouse Savannah River Company
Aiken, South Carolina 29808
M. B. Goli
Mississippi Valley State University
Itta Bena, MS 38941
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
The toxicity of gadolinium to algae and bacteria was determined as part of an effort to develop a biological process to purify drums containing spent nuclear reactor heavy water moderator (D2O). This water was contaminated with high concentrations of gadolinium nitrate, a chemical used as a neutron poison during former nuclear reactor operations at the Savannah River Site (SRS) near Aiken, SC. Nuclear reactors were operated for approximately 30 years at the SRS to produce nuclear weapons materials for national defense. Throughout this period, a heavy water solution of gadolinium nitrate was utilized in a standby emergency shutdown system that could inject this chemical into the reactor moderator coolant water. The chemical was used for this purpose because the high neutron absorption cross sections of some gadolinium isotopes make gadolinium salts such as GdNO3 effective in controlling nuclear activity in aqueous systems (Gilbert et al. 1985; Rodenas et al. 1990). The use of this practice resulted in a large inventory of this degraded heavy water containing gadolinium nitrate.
Microbiological and chemical studies were initiated to evaluate the potential use of bacteria and algae for water purification of the drums. Since metals are often toxic to microbes when present at concentrations substantially higher than natural environmental levels, it was hypothesized that Gd may be toxic to selected microorganisms (algae and bacteria) at the very high concentrations (average 80,000 mg/L, maximum 259,000 mg/L) present in most of the drums. Two principal components of the study included: (1) chemical and microbiological characterization of representative drums, and (2) an evaluation of the toxicity of gadolinium to selected species of algae. In addition to wastewater from nuclear production reactor operations, gadolinium waste is also generated from medical applications, especially MRI, and various electronic components including CD disks. Despite growing and widespread usage of this rare element, there is a paucity of information on the toxicity of gadolinium.
Materials and Methods
Eight drums of the wastewater representing a wide range of Gd concentrations and other chemical conditions (based on prior sampling) were selected for the present study. Conditions included relatively high and low values for gadolinium, pH, conductivity and % D2O. One-liter samples were collected from each drum. Gadolinium (mg/L) was measured by inductively coupled argon plasma spectroscopy, pH (units) by a combination electrode, D2O (mole %) by infrared spectroscopy, conductivity (m mhos/cm) by the electrolytic method and NO3 by ion chromatography.
Microbiological processing and analysis techniques included plate counts and microscopical counts. Duplicate spread plates were prepared using 10 m L and 100 m L inoculums taken from each drum sample to determine colony forming units (Balkwell, 1989). The plates were prepared and incubated at room temperature prior to enumeration. Total direct bacteria counts of cells were performed by spotting 50 m L of well-mixed water onto toxoplasmosis slides (Santo Domingo, et al. 1998). Nutrient additions were made to the samples by first transferring 7.0 mL of each drum sample into sterile 15-mL centrifuge tubes. Then, 250 uL of a glucose solution was added to each vial to yield a final glucose concentration of 0.34%. Capped vials were placed on a rotating shaker for 72 hours prior to being analyzed by the direct count method.
Three experiments were conducted to evaluate the toxicity of gadolinium to algae. Samples were prepared in triplicate. In the initial experiment, four species of algae were inoculated into standard culture media with and without GdNO3 substituted for conventional nitrate (e.g., NaNO3) to provide GdNO3 at concentrations resulting in Gd concentrations of 0, 10, 100, 1,000, 10,000, 80,000 and 260,000 mg/L (thus, bracketing the quantities in all of the drums requiring treatment). Gd and NO3 concentrations of the media were measured at the start of the experiment and after one and two weeks. Algal cultures included Chlorella pyrenoidosa, Scenedesmus quadricauda, Closterium sp. and Cyanidium caldarum. All four strains were obtained from the Carolina Biological Supply Company, Burlington, NC. Chlorella and Scenedesmus were grown in modified Bold Basal (BB) medium (Nichols and Bold 1965). Closterium was grown in Alga Gro Freshwater Medium and Cyanidium in Doemel's Cyanidium medium (Carolina Biological Supply Co., 1978.)
Culture media was prepared in 25 mL batches in 50 mL flasks prior to being autoclave sterilized. Flasks were subsequently inoculated with 600 m L of algal culture. The flasks were then placed in a Pschrotherm shaker/incubator (New Brunswick Scientific) for two weeks at 20°C, with a 12 h light (200 m E-2 s-1):12 h dark illumination regime and 100 rpm rotation. Control flasks, not inoculated with algae but otherwise treated identically with all treatment conditions, were examined for nitrate and gadolinium concentrations at the start and conclusion of all three experiments.
Densities of live algae were determined by examining aliquots from the flasks with an inverted microscope using fluorescence microscopy which allows the differentiation of live and dead cells by the detection of chlorophyll fluorescence when cells are subjected to excitation by blue or green light (Wilde and Fliermans,1979; Wood et al. 1985). Density estimates were made by randomly counting fields until minimums of 400 cells per sample were tabulated. Counts were performed after incubation periods of one- and two-weeks. Aliquots (250 m l) were collected from the flasks for microscopical observations and the remaining liquid was filtered (0.45 m m pore size). The filters were labeled, dried and photographed. The relative amount of algae observed on the dried filters was compared to the quantitative count data.
A second experiment was conducted to further elucidate the toxicity of GdNO3 to algae. Based on the results of Experiment 1, the range of Gadolinium that the algae were exposed to was decreased and two additional algal strains were evaluated. These were isolates of the species, Mastigocladus laminosus, a thermophilic blue green alga derived from SRS reactor effluent streams and cultured in Medium ND (Castenholz 1982). Three different pH levels, 3.5, 4.5, and 5.5 were also compared. Algae were enumerated at the start of the experiment and at one- and two-week intervals.
A third experiment was conducted to the comparative toxicity of GdNO3, NaNO3, KNO3, and NH4NO3 to algae. Modified BB Medium was formulated to contain 10, 100, 1,000, and 10,000 mg/L of Gd as GdNO3, Na as NaNO3, K as KNO3, and NH4 as NH4NO3.. Triplicate samples containing each medium were inoculated with 600 m l of a Chlorella vulgaris suspension and algal concentrations were determined immediately and after one week of incubation under Pschrotherm incubator conditions previously described.
Results and Discussion
Complete results of chemical and microbiological analyses of the drum samples are shown in Table 1. The samples with the highest gadolinium concentrations generally displayed proportionally higher concentrations of NO3, and conductivity.
Drums with relatively high gadolinium concentrations also tended to have the least bacteria. Although only three of the drum samples produced culturable bacteria on 1% PTYG agar. Total direct counts showed that some bacteria were present in all drums. This indicates that at least some bacteria are capable of tolerating > 99% D2O with nitrate and gadolinium concentrations greater than 200,000 mg/L (Table 1).
The microbial density increased in six of the eight drum samples, as measured using the direct count method, when glucose was added to the drum samples. Bacteria densities increased more than two orders of magnitude with nutrient addition in three of the samples. All of these contained less than 100 mg/L
gadolinium. Cells in many of these samples were larger and/or in the process of dividing when viewed under the microscope indicating the presence of actively growing cells. The largest increase in cellular density occurred with the sample from drum #15435, which had a very low gadolinium concentration. This sample also had the largest number of colony forming units as determined by the spread plate method. The addition of an organic carbon source, glucose, appeared to stimulate the growth of the bacteria that were already present in the samples.
Table 1. Characterization Data: Drums of Spent Moderator Water
|
Drum # |
18156 |
15208 |
15817 |
4600 |
15793 |
15861 |
15435 |
13749 |
|
Microbiological data |
||||||||
|
Direct Count Cells/ml |
1.24E+05 |
6.14E+05 |
4.74E+04 |
1.29E+05 |
5.57E+04 |
8.25E+03 |
1.22E+06 |
9.28E+03 |
|
Spread Plate CFU/ml |
ND |
2.00E+01 |
ND |
ND |
ND |
ND |
6.35E+03 |
6.20E+02 |
|
72 hr. Nutrient Cell/ml |
3.44E+05 |
2.03E+03 |
3.80E+04 |
2.08E+05 |
1.43E+06 |
1.38E+06 |
>1.0E+08 |
2.44E+04 |
|
Chemical data |
||||||||
|
Gd (mg/L) |
202500 |
203800 |
0.09 |
142 |
97 |
0.5 |
0.26 |
6118 |
|
Nitrate (mg/L) |
183936 |
279985 |
<1 |
179 |
63 |
<1 |
<1 |
5954 |
|
pH |
1.63 |
5.36 |
6.14 |
3.62 |
6.79 |
6.34 |
5.59 |
6.66 |
|
D2 0% |
78.17 |
99.59 |
98.73 |
98.54 |
99.65 |
99.47 |
99.17 |
2.99 |
|
Cond. (µmhos) |
84400 |
97500 |
4.07 |
394 |
199 |
1.4 |
10.8 |
9020 |
|
Nitrate (mg/L) |
239963 |
241503 |
0.1 |
168 |
115 |
0.6 |
0.3 |
7250 |
Although microbiological sampling demonstrated the presence of microorganisms in all eight of the drums sampled it also suggested that gadolinium has a toxic effect on growth since substantial growth could only be enhanced by nutrient addition in three of four drums with relatively low (<100 mg/L) gadolinium concentrations. No significant increase in microbial density occurred in drums with Gadolinium concentrations of >100 mg/L with the addition of nutrients (glucose) to the drum water.
The last row in Table 1 shows the expected (calculated) levels of nitrate based on the gadolinium measurements and the molecular weights of the elements in the Gd(NO3)3.6H2O compound used in the reactor process where the water in the drums originated. Measured concentrations of Gd, NO3, and conductivity were strongly correlated with each other. Expected (calculated) and measured values of nitrate were generally similar. There was no clear evidence from these analyses that bacteria were reducing nitrate in the drums since some of the drums had measured nitrate levels higher than expected values based entirely on the stoichiometry (Table 1).
Observations of the relative amounts of algae on the dried filters following incubation led to the same conclusions regarding growth as the quantitative counts utilizing the microscope. Thus, labeling and photographing of the dried filters provided a quick screening method to qualitatively observe and document relative quantities of algal growth. This procedure eliminates the need to conduct laborious microscopic counts for all samples, particularly those where algal growth is clearly inferior based on naked eye assessments. Use of this technique allowed us to quickly eliminate several algal strains and media conditions from further consideration during the initial screening phase of process development.
Chlorella vulgaris was more tolerant to gadolinium nitrate than Scenedesmus quadricauda or Cyanidium caldarum (Figure 1). The microscopical examinations also revealed some viable algal cells at gadolinium concentrations up to 260,000 mg/L. However, growth was greatly impeded at gadolinium concentrations between 100 mg/L and 1,000 mg/L. The growth data in Figure 1 represent the change in algal populations between the first and second week of inoculation in the media. Figure 2 shows the amount of Chlorella growth in each of two consecutive 1-week periods when exposed to various concentrations of Gd and pH with BB media. At all pH conditions tested, algal growth decreased dramatically between 100 mg/L and 1,000 mg/L. These data support the conclusion that algal growth is inhibited at concentrations in this range.
At pH 4.5 and 5.5, Chlorella grew much better when gadolinium concentrations were 0 and 100 mg/L compared to a gadolinium concentration of 10 mg/L (Figure 2) (This was also evident in Figure 1). This result, which may appear as a data anomaly at first glance, can be explained by the methodology. The 0 mg/L gadolinium samples were prepared with the standard modified BB media formulation which contains 182.5 mg/L of nitrate as NaNO3; whereas, the formulations for 10 to 10,000 mg/L gadolinium samples were prepared by substituting Gd/NO3 in place of sodium nitrate. Thus, the 0, 10, and 100 mg/L gadolinium formulations contained 182.5, 11.9, and 118.5 mg/L nitrate, respectively and the lower growth at 10 mg/L Gd, relative to the 0 mg/L and 100 mg/L concentrations was most likely due to nitrate limitation rather than gadolinium toxicity.
Growth was substanially higher in the second week of incubation than in the first week with the exception of the 10 mg/L Gd exposures at pH 4.5 and pH 5.5. Once again, the lower growth at 10 mg/L Gd is thought to be due to the depletion of nitrate after the first week. The higher growth in week #2 compared to week #1 in the other samples may represent adaptation to the gadolinium in the medium. Figure 3 shows the growth of Chlorella in modified BB media containing nitrate salts prepared with gadolinium, sodium, potassium and ammonium at concentrations of 0, 10, 100, 1,000 and 10,000 of the cationic portion of each compound. Gadolinium appears to stimulate growth at 10 mg/L but results in reduced growth at the higher concentrations. The highest growth occurred with sodium at 100mg/L. However, at the highest concentration tested (100,00 mg/L), potassium resulted in the best growth.

Figure 1. Growth of three species of algae at various concentrations of gadolinium
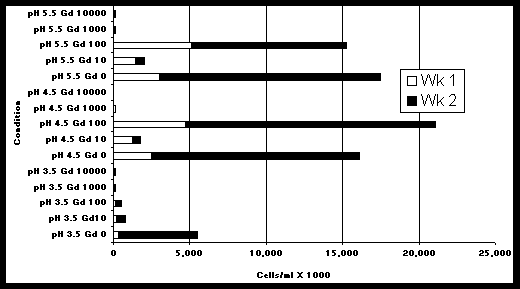
Figure 2. Growth of Chlorella during exposure to various pH and gadolinium concentrations (mgL)
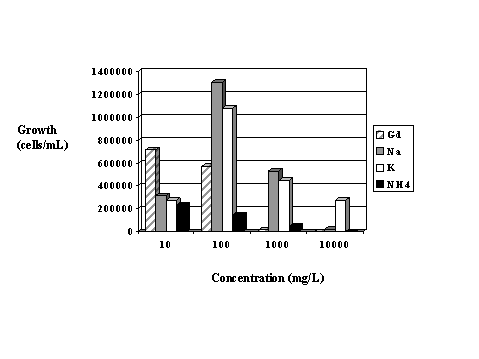
Figure 3. Comparative algal growth in four nitrate salt solutions
Overall, it was concluded that although some microbes can survive at extremely high Gd concentrations, gadolinium is generally toxic to algae and bacteria at concentrations between 100 and 1,000 mg/L. It was also demonstrated that gadolinium is more toxic to algae than potassium or sodium.
Acknowledgments
The authors are grateful to Marilyn Franck, Mary Anne Johnson, Fatina Washburn, Sherold Johnson, Mike Polochko, Robert Ray and Charlie Varner for providing technical assistance on this project. Funding for the work was provided under Contract DE-AC09-96SR18500 with the U.S. Department of Energy.
References