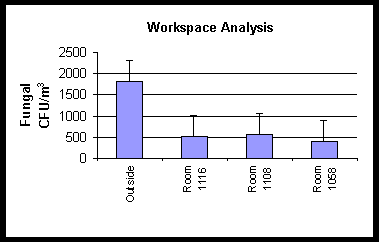
Figure 1. This graph shows viable fungal densities for a workspace
analysis in a building where fungal amplification
could not be proven by air samples.
WSRC-MS-2000-00310
An Improved Method for Direct Fungi Identification and Enumeration
M.M. Franck, R.L. Brigmon, P.C. McKinsey, M.A. Heitkamp, and
C.B. Fliermans
Westinghouse Savannah River Company
Aiken, South Carolina
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
This report has been reproduced directly from the best available copy.
Available for sale to the public, in paper, from: U.S. Department of Commerce, National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161, phone: (800) 553-6847, fax: (703) 605-6900, email: orders@ntis.fedworld.gov online ordering: http://www.ntis.gov/support/ordering.htm
Available electronically at http://www.osti.gov/bridge/
Available for a processing fee to U.S. Department of Energy and its contractors, in paper, from: U.S. Department of Energy, Office of Scientific and Technical Information, P.O. Box 62, Oak Ridge, TN 37831-0062, phone: (865 ) 576-8401, fax: (865) 576-5728, email: reports@adonis.osti.gov
Abstract
An improved method for direct fungal identification and enumeration in air and surface samples was developed for use at the Department of Energy’s Savannah River Site (SRS), Aiken, SC. Direct microscopic examination of fungal hyphae and conidia is often difficult for indoor samples due to debris, including pollen and fluorescent textile fibers. Therefore, a staining method incorporating FUN-1 (Molecular Probes, Eugene, OR), Fluorescent Brightener 28 (Sigma Chemical Co., St. Louis, MO), and potassium hydroxide was developed to directly examine microorganisms in air and physical samples. The sampling included environmental samples from several buildings using the Andersen 6-Stage Viable Particle-Sizing Air Sampler (Smyrna, Georgia), and direct surface sampling where fungal growth was suspected. Split samples showed the new staining method was more effective in detecting and distinguishing fungal structures collected during sampling and also enhanced clarity of structures of fungal isolates. Application of this technique has increased the speed and sensitivity of fungi detection for workspace monitoring. This method was applied to workspace assessments and has increased understanding of the relationships between fungal growth on surfaces, airborne fungi, environmental factors and overall workspace assessment.
Introduction
Exposure to indoor air contaminated by fungi and their associated structures commonly occurs, and can cause allergic, toxic and/or irritant symptoms and diseases. The occurrence of specific microorganisms in air has been associated with a number of human health concerns ranging from dermatitis, central nervous disorders, and immune dysfunction to fatal pulmonary disease. The reported association between elevated concentrations of airborne fungal structures and human illness has served to focus public and scientific attention on the potential health risks associated with fungal contaminated indoor environments. As part of our efforts to address health concerns, microbiological workspace assessments are underway at SRS. This fungal staining technique incorporating FUN-1, Fluorescent Brightener 28, and hydrogen peroxide was developed to improve these microbial assessments.
Materials and Methods
Air sampling was conducted with an Andersen 6-Stage Air Sampler, which consisted of six aluminum stages connected to a vacuum pump. The sampling time ranged from one to five minutes. The samplers were placed at desk height of approximately thirty centimeters for all indoor air sample collections. Outdoor samples were taken close to the building HVAC unit air intake. 3% glucose malt extract agar (MEA) was used for viable fungal counts. 70% isopropyl alcohol wipes was used to decontaminate stages between each sample location. Following exposure all agar plates were taped, bagged, and transported to the laboratory. The fungal collection plates were incubated for one week at 25°C. Viable fungal colonies were enumerated on a Leica Darkfield Quebec Colony Counter (Buffalo, NY). The final average CFU/m3 viable counts for each area tested were taken from Andersen plates that were examined and counted one week later.
Adhesive tape impressions for microscopic examination were taken from areas having suspected fungal contamination on ceiling tiles, baseboards, wallboard, air vents and air returns. Tape was lightly pressed onto suspected fungal growth surfaces and mounted onto microscope slides. Collected tape samples were placed in sterile 50mL tubes for transport to the laboratory. The staining method employed consisted of 10 m Mol FUN-1ä by Molecular Probes, 1% Fluorescence Brightener 28, Sigma-Aldrich Chemical Co. and 5% potassium hydroxide in nano pure water. The stain solution is applied directly to the tape mounted on the slide, a cover slip applied, and the slide is incubated 20 minutes in the dark. Tape impressions taken of fungal colonial growth on Petri plates were mounted and stained as above. View the stained slide with an epifluorescent DAPI filter (with an excitation wavelength of 365 nm and emission wavelength of 425 nm.).
Results and Discussion
The FUN-1ä /Fluorescence Brightener 28 stain was found to be useful when doing workspace mycological analyses. When air samples are taken, an outside air sample is also performed to serve as a point of reference. Normally, the indoor fungal CFU/m3 densities should be much lower than the outside fungal density. Figure 1 is a graph of the results of one building tested that illustrates the point. The data presented indicates that the building did not have a fungal amplification problem, but numerous fungal hyphae and conidia could be seen on microscopic examination of tape impressions of stained walls and upholstery. The FUN-1ä /Fluorescence Brightener 28 stain was instrumental in differentiating fungal structures from industrial fibers, insect parts and hair.

Figure 1. This graph shows viable fungal densities for a workspace
analysis in a building where fungal amplification
could not be proven by air samples.
In Figure 2 a roof leak had caused water damage in Rooms 1035 and 1036. When
air samples were performed, it was found that the affected rooms did have elevated
fungal densities as compared to the outside densities. These rooms were closed
for renovation.
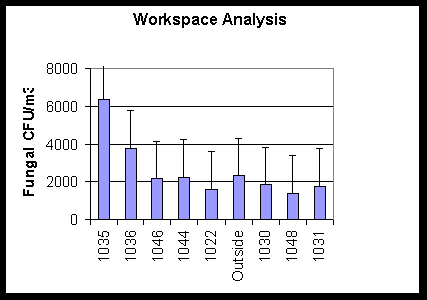
Figure 2. This graph shows viable fungal densities for a
workspace analysis in a
building where fungal amplification is suspected in one room
as compared to reference rooms and an outside air sample.
Two months later another air sample in this building was performed, and the results can be seen in Figure 3. The closed room, 1035, had lowered fungal densities as compared to the previous sampling period and also to the outside fungal density sampled the same day. The room had not been disturbed, so the reduction in the fungal densities could be attributed to the decreased physical activity due to room closure.
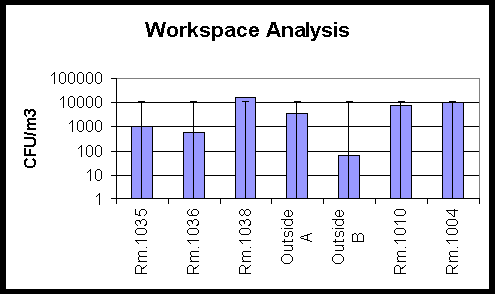
Figure 3. This graph shows fungal densities from the same
building as
in Figure 2, taken two months later. The room of concern
had been closed during the two-month interval.
Small holes were cut in the walls of the rooms affected by the water leak. The holes were 3" x 5" under the baseboards of these rooms. Tape impressions of the wallboard within the holes showed extensive fungal amplification. The stain technique described was used effectively to demonstrate various fungal structures. The presence of the fungal amplification within the wall spaces could be a source for intermittent aerosol contamination. Several pictures on this poster come from this building.
Figure 4 and Figure 5 are pictures of tape impressions taken from visible fungal
growth seen on water damaged wallboard. These slides were stained with the FUN-1ä
/Fluorescence Brightener 28 method described above. The fungal structures are
highlighted and contrast quite well against the blue background of the DAPI
filter.
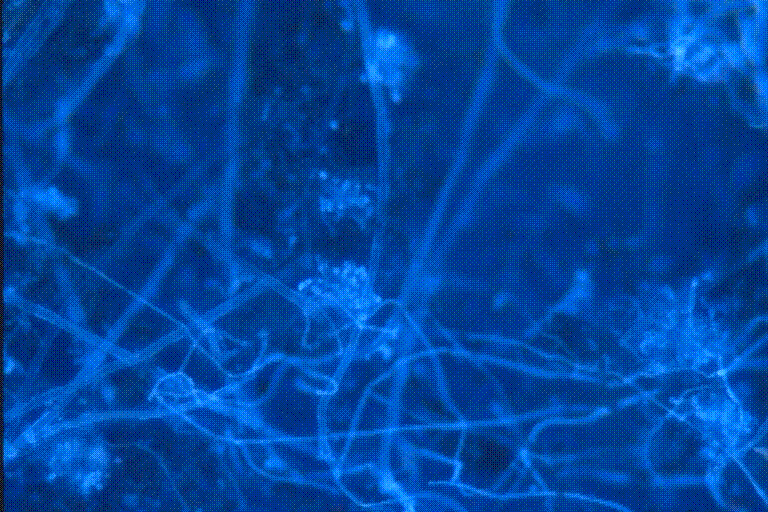
Figure 4. Tape impression of fungal contamination seen on wallboard damaged
by water.
This slide was stained with the FUN-1ä /Fluorescent
Brightener 28 stain
described. This fungal growth was seen in the building described
in Figure 2 and Figure 3.
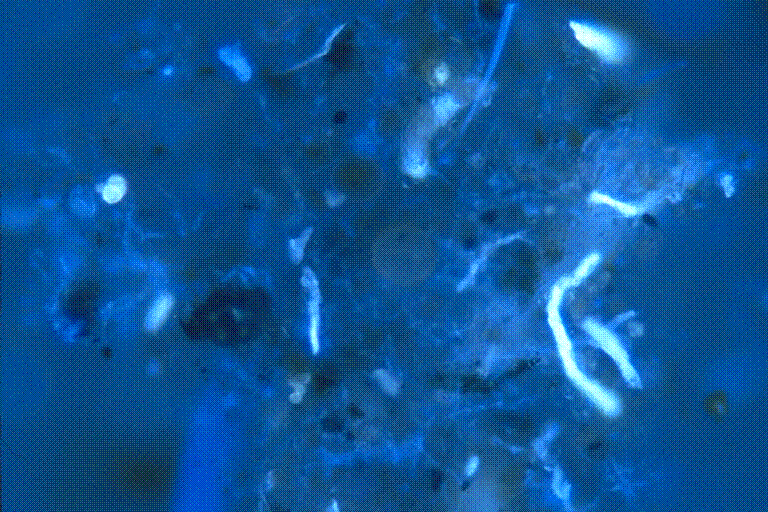
Figure 5. Tape impression of wallboard fungal contamination. This slide
was
stained with the FUN-1ä /Fluorescent Brightener
28 stain
described. Dirt and debris can be seen in this picture that
doesn’t stain. The fungal structures are well highlighted
and stand out from the background.
Figure 6 and Figure 7 are pictures of tape impressions also taken from visible fungal growth seen on water damaged wallboard and stained with Lactophenol Cotton Blue. Figure 6 shows a tape impression of conidia from Stachybotrys chartarum contamination. Figure 6 and Figure 7 slides are stained with Lactophenol Cotton Blue and viewed by bright field microscopy.
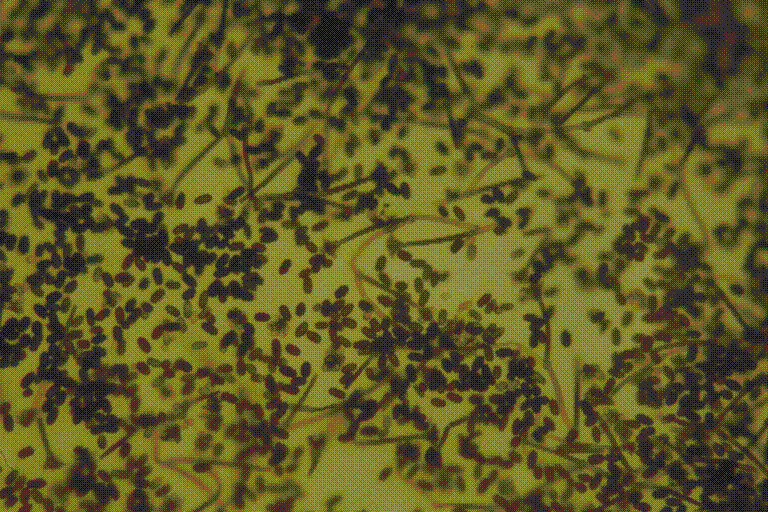
Figure 6. Tape impression of fungal contamination seen on water damaged
wallboard. This fungal growth was seen in the building described
in Figures 2 and 3. This slide was stained with Lactophenol
Cotton Blue. The conidia and mycelia are characteristic
of Stachybotrys chartarum.

Figure 7. Tape impression of fungal contamination seen on water-damaged
wallboard.
This fungal growth was seen in the building described in Figure 2 and
Figure 3. This slide was stained with Lactophenol Cotton Blue.
Figure 8 shows the plates that were used in the Andersen 6 Stage Viable Particle Sizing Sampler used to collect the air samples in Figure 2. The pore sizes of the 6 stages of the air sampler simulate the sizes of the air passages of the human respiratory system. Figure 9 shows fungal contamination behind wallpaper that has been water damaged.
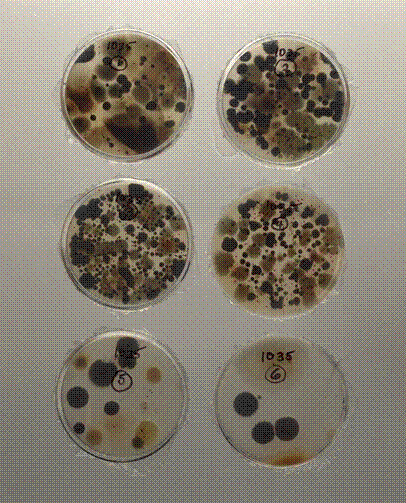
Figure 8. Glass plates of air samples collected with the
Andersen 6 Stage Viable Particle
Sizing Sampler used to collect the air samples in Figure 2. Each
stage contains 400 orifices with diameters ranging from
1.81 mm on the first stage to 0.25 mm on
the sixth stage.

Figure 9. Gross fungal contamination can be
seen here on
water-damaged wallpaper and wallboard.
Conclusion
This method (FUN-1ä /Fluorescence Brightener 218) was designed for direct evaluation of environmental samples for fungal contamination. This method was clearly successful in demonstrating fungal contamination where ambient air sampling proved inadequate for full indoor workspace analyses. When this method was applied to slides with high background, problematic fungal structures were highlighted and easily differentiated. Microscopy of fungal materials stained with the FUN-1ä /Fluorescence Brightener method was far superior to FUN-1ä, Fluorescence Brightener, or Lactophenol Cotton Blue methods by themselves.
This simple 1 step preparation of environmental samples for fluorescence microscopy described should be valuable for indoor workspace mycological evaluations. Although a prospective study would be necessary to evaluate the full diagnostic potential of this method, the marked improved sensitivity observed agrees with confirmatory results. This technique could also have other applications including human and veterinary mycology examinations.